Gut-brain circuits for fat preference
- PMID: 36070796
- PMCID: PMC9605869
- DOI: 10.1038/s41586-022-05266-z
Gut-brain circuits for fat preference
Abstract
The perception of fat evokes strong appetitive and consummatory responses1. Here we show that fat stimuli can induce behavioural attraction even in the absence of a functional taste system2,3. We demonstrate that fat acts after ingestion via the gut-brain axis to drive preference for fat. Using single-cell data, we identified the vagal neurons responding to intestinal delivery of fat, and showed that genetic silencing of this gut-to-brain circuit abolished the development of fat preference. Next, we compared the gut-to-brain pathways driving preference for fat versus sugar4, and uncovered two parallel systems, one functioning as a general sensor of essential nutrients, responding to intestinal stimulation with sugar, fat and amino acids, whereas the other is activated only by fat stimuli. Finally, we engineered mice lacking candidate receptors to detect the presence of intestinal fat, and validated their role as the mediators of gut-to-brain fat-evoked responses. Together, these findings reveal distinct cells and receptors that use the gut-brain axis as a fundamental conduit for the development of fat preference.
© 2022. The Author(s).
Conflict of interest statement
C.S.Z. is a scientific co-founder and advisor of Kallyope. The other authors declare no competing interests.
Figures
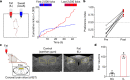

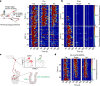

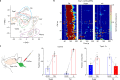











Comment in
-
Gut-brain axis key to fat preference.Nat Rev Gastroenterol Hepatol. 2022 Nov;19(11):687. doi: 10.1038/s41575-022-00693-9. Nat Rev Gastroenterol Hepatol. 2022. PMID: 36168048 No abstract available.
References
-
- Zhang Y, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. - PubMed
-
- Food Balances (2010–)https://www.fao.org/faostat/en/#data/FBS (Food and Agriculture Organization of the United Nations, 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

