Microfluidics for Neuronal Cell and Circuit Engineering
- PMID: 36070858
- PMCID: PMC9523714
- DOI: 10.1021/acs.chemrev.2c00212
Microfluidics for Neuronal Cell and Circuit Engineering
Abstract
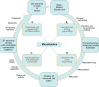
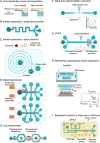
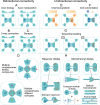
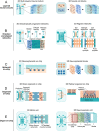
The widespread adoption of microfluidic devices among the neuroscience and neurobiology communities has enabled addressing a broad range of questions at the molecular, cellular, circuit, and system levels. Here, we review biomedical engineering approaches that harness the power of microfluidics for bottom-up generation of neuronal cell types and for the assembly and analysis of neural circuits. Microfluidics-based approaches are instrumental to generate the knowledge necessary for the derivation of diverse neuronal cell types from human pluripotent stem cells, as they enable the isolation and subsequent examination of individual neurons of interest. Moreover, microfluidic devices allow to engineer neural circuits with specific orientations and directionality by providing control over neuronal cell polarity and permitting the isolation of axons in individual microchannels. Similarly, the use of microfluidic chips enables the construction not only of 2D but also of 3D brain, retinal, and peripheral nervous system model circuits. Such brain-on-a-chip and organoid-on-a-chip technologies are promising platforms for studying these organs as they closely recapitulate some aspects of in vivo biological processes. Microfluidic 3D neuronal models, together with 2D in vitro systems, are widely used in many applications ranging from drug development and toxicology studies to neurological disease modeling and personalized medicine. Altogether, microfluidics provide researchers with powerful systems that complement and partially replace animal models.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Microfluidic Brain-on-a-Chip: Perspectives for Mimicking Neural System Disorders.Mol Neurobiol. 2019 Dec;56(12):8489-8512. doi: 10.1007/s12035-019-01653-2. Epub 2019 Jul 1. Mol Neurobiol. 2019. PMID: 31264092 Free PMC article. Review.
-
Fluidic circuit board with modular sensor and valves enables stand-alone, tubeless microfluidic flow control in organs-on-chips.Lab Chip. 2022 Mar 15;22(6):1231-1243. doi: 10.1039/d1lc00999k. Lab Chip. 2022. PMID: 35178541 Free PMC article.
-
Neuronal circuits on a chip for biological network monitoring.Biotechnol J. 2021 Jul;16(7):e2000355. doi: 10.1002/biot.202000355. Epub 2021 May 31. Biotechnol J. 2021. PMID: 33984186
-
Engineering Cardiac Tissue for Advanced Heart-On-A-Chip Platforms.Adv Healthc Mater. 2024 Jan;13(1):e2301338. doi: 10.1002/adhm.202301338. Epub 2023 Aug 3. Adv Healthc Mater. 2024. PMID: 37471526 Review.
-
Fitting tissue chips and microphysiological systems into the grand scheme of medicine, biology, pharmacology, and toxicology.Exp Biol Med (Maywood). 2017 Oct;242(16):1559-1572. doi: 10.1177/1535370217732765. Exp Biol Med (Maywood). 2017. PMID: 29065799 Free PMC article.
Cited by
-
Impact of Neurons on Patient-Derived Cardiomyocytes Using Organ-On-A-Chip and iPSC Biotechnologies.Cells. 2022 Nov 25;11(23):3764. doi: 10.3390/cells11233764. Cells. 2022. PMID: 36497024 Free PMC article.
-
Influence of microgravity on spontaneous calcium activity of primary hippocampal neurons grown in microfluidic chips.NPJ Microgravity. 2024 Feb 6;10(1):15. doi: 10.1038/s41526-024-00355-x. NPJ Microgravity. 2024. PMID: 38321051 Free PMC article.
-
3D in vitro modeling of neural microenvironment through a multi-scaffold assembly approach.Mater Today Bio. 2025 Jul 14;33:102086. doi: 10.1016/j.mtbio.2025.102086. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40727076 Free PMC article.
-
Brain-on-a-chip: an emerging platform for studying the nanotechnology-biology interface for neurodegenerative disorders.J Nanobiotechnology. 2024 Sep 18;22(1):573. doi: 10.1186/s12951-024-02720-0. J Nanobiotechnology. 2024. PMID: 39294645 Free PMC article. Review.
-
Portrait of intense communications within microfluidic neural networks.Sci Rep. 2023 Jul 29;13(1):12306. doi: 10.1038/s41598-023-39477-9. Sci Rep. 2023. PMID: 37516789 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

