Efficacy and Safety of Botulinum Toxin Type A (NABOTA) for Post-stroke Upper Extremity Spasticity: A Multicenter Phase IV Trial
- PMID: 36070998
- PMCID: PMC9452292
- DOI: 10.5535/arm.22061
Efficacy and Safety of Botulinum Toxin Type A (NABOTA) for Post-stroke Upper Extremity Spasticity: A Multicenter Phase IV Trial
Abstract
Objective: To evaluate the efficacy and safety of Daewoong botulinum toxin type A (NABOTA) after its launch in South Korea.
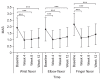
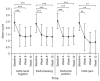
Methods: This prospective, multicenter, open-label phase IV clinical trial included 222 patients with stroke. All patients visited the clinic at baseline and at weeks 4, 8, and 12 after injection of upto 360 units of NABOTA into the wrist, elbow, and finger flexor muscles at the first visit. The primary outcome was the change in Modified Ashworth Scale (MAS) score for the wrist flexor muscles between baseline and week 4. The secondary outcomes were the changes in MAS, Disability Assessment Scale (DAS), and Caregiver Burden Scale (CBS) scores between baseline and each visit, and the Global Assessment Scale (GAS) score at week 12.
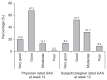
Results: There was a statistically significant decrease in the MAS score for the wrist flexors between baseline and week 4 (-0.97±0.66, p<0.001). Compared with baseline, the MAS, DAS and CBS scores improved significantly during the study period. The GAS was rated as very good or good by 86.8% of physicians and by 60.0% of patients (or caregivers). The incidence of adverse events was 14.4%, which is smaller than that in a previous trial.
Conclusion: NABOTA showed considerable efficacy and safety in the management of upper limb spasticity in stroke patients.
Keywords: Botulinum toxin type A; Clinical trial; Muscle spasticity; Phase 4; Safety; Stroke; Treatment efficacy.
Conflict of interest statement
The corresponding author of this manuscript is an editor of Annals of Rehabilitation Medicine. The corresponding author did not engage in any part of the review and decision-making process for this manuscript.
Figures

Similar articles
-
Efficacy and safety of NABOTA in post-stroke upper limb spasticity: a phase 3 multicenter, double-blinded, randomized controlled trial.J Neurol Sci. 2015 Oct 15;357(1-2):192-7. doi: 10.1016/j.jns.2015.07.028. Epub 2015 Jul 21. J Neurol Sci. 2015. PMID: 26233808 Clinical Trial.
-
A Randomized, Double-Blind, Active Control, Multicenter, Phase 3 Study to Evaluate the Efficacy and Safety of Liztox® versus Botox® in Post-Stroke Upper Limb Spasticity.Toxins (Basel). 2023 Dec 12;15(12):697. doi: 10.3390/toxins15120697. Toxins (Basel). 2023. PMID: 38133201 Free PMC article. Clinical Trial.
-
Efficacy and Safety of MT10107 (Coretox) in Poststroke Upper Limb Spasticity Treatment: A Randomized, Double-Blind, Active Drug-Controlled, Multicenter, Phase III Clinical Trial.Arch Phys Med Rehabil. 2020 Sep;101(9):1485-1496. doi: 10.1016/j.apmr.2020.03.025. Epub 2020 Jun 1. Arch Phys Med Rehabil. 2020. PMID: 32497599 Clinical Trial.
-
Botulinum Toxin Type A for Upper Limb Spasticity in Poststroke Patients: A Meta-analysis of Randomized Controlled Trials.J Stroke Cerebrovasc Dis. 2020 Jun;29(6):104682. doi: 10.1016/j.jstrokecerebrovasdis.2020.104682. Epub 2020 Apr 15. J Stroke Cerebrovasc Dis. 2020. PMID: 32305277 Review.
-
Botulinum toxin treatment of adult spasticity : a benefit-risk assessment.Drug Saf. 2006;29(1):31-48. doi: 10.2165/00002018-200629010-00003. Drug Saf. 2006. PMID: 16454533 Review.
References
-
- Kuo CL, Hu GC. Post-stroke spasticity: a review of epidemiology, pathophysiology, and treatments. Int J Gerontol. 2018;12:280–4.
-
- Sunnerhagen KS, Olver J, Francisco GE. Assessing and treating functional impairment in poststroke spasticity. Neurology. 2013;80(3 Suppl 2):S35–44. - PubMed
-
- Kim CS, Song KY, Min KM, An YD, inventors. Method for production of botulinum toxin. 2015/016462 (KR2014/004003) World patent WO. 2015 Feb 5;
-
- Kim CS, Jang WS, Son IP, Nam SH, Kim YI, Park KY, et al. Electrophysiological study for comparing the effect of biological activity between type A botulinum toxins in rat gastrocnemius muscle. Hum Exp Toxicol. 2013;32:914–20. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

