Construction of a culture protocol for functional bile canaliculi formation to apply human iPS cell-derived hepatocytes for cholestasis evaluation
- PMID: 36071090
- PMCID: PMC9452549
- DOI: 10.1038/s41598-022-19469-x
Construction of a culture protocol for functional bile canaliculi formation to apply human iPS cell-derived hepatocytes for cholestasis evaluation
Abstract
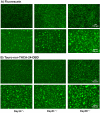
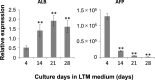
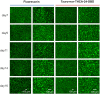
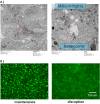
Cholestatic toxicity causes the failure of pharmaceutical agents during drug development and, thus, should be identified at an early stage of drug discovery and development. The formation of functional bile canaliculi in human hepatocytes is required for in vitro cholestasis toxicity tests conducted during the early stage of drug development. In this study, we investigated the culture conditions required for the formation of bile canaliculi using human-induced pluripotent stem cell-derived hepatocytes (hiPSC-Heps). When hiPSC-Heps were sandwich-cultured under the condition we established, extended bile canaliculi were formed on the whole well surfaces. Biliary efflux transporters were localized in the formed bile canaliculi structures which had junctional complexes. After the model substrates of the biliary efflux transporters were taken up into cells, their subsequent excretion into the bile canaliculi was observed and was found to be impeded by each inhibitor of the biliary efflux transporter. These findings suggest that bile canaliculi have transporter-specific bile excretion abilities. We will continue to study the application of this culture protocol to cell-based cholestasis assay system. As a result, the culture protocol could lead to a highly predictable, robust cell-based cholestasis assay system because it forms functional bile canaliculi reproducibly and efficiently.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Morales M, Vélez L, Muñoz M. Hepatotoxicity: A drug-induced cholestatic pattern. Rev. Colomb. Gastroenterol. 2016;31(1):36–47. doi: 10.22516/25007440.71. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

