Carbon dioxide-enhanced metal release from kerogen
- PMID: 36071133
- PMCID: PMC9452497
- DOI: 10.1038/s41598-022-19564-z
Carbon dioxide-enhanced metal release from kerogen
Abstract
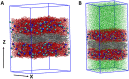
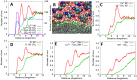
Heavy metals released from kerogen to produced water during oil/gas extraction have caused major enviromental concerns. To curtail water usage and production in an operation and to use the same process for carbon sequestration, supercritical CO2 (scCO2) has been suggested as a fracking fluid or an oil/gas recovery agent. It has been shown previously that injection of scCO2 into a reservoir may cause several chemical and physical changes to the reservoir properties including pore surface wettability, gas sorption capacity, and transport properties. Using molecular dynamics simulations, we here demonstrate that injection of scCO2 might lead to desorption of physically adsorbed metals from kerogen structures. This process on one hand may impact the quality of produced water. On the other hand, it may enhance metal recovery if this process is used for in-situ extraction of critical metals from shale or other organic carbon-rich formations such as coal.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Igunnu ET, Chen GZ. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2012;9(3):157–177. doi: 10.1093/ijlct/cts049. - DOI
-
- Al-Ghouti MA, Al-Kaabi MA, Ashfaq MY, Da’na DA. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019;28:222–239. doi: 10.1016/j.jwpe.2019.02.001. - DOI
-
- Chong L, Sanguinito S, Goodman AL, Myshakin EM. Molecular characterization of carbon dioxide, methane, and water adsorption in micropore space of kerogen matrix. Fuel. 2021;283:119254. doi: 10.1016/j.fuel.2020.119254. - DOI
LinkOut - more resources
Full Text Sources

