Engineered marble-like bovine fat tissue for cultured meat
- PMID: 36071206
- PMCID: PMC9452530
- DOI: 10.1038/s42003-022-03852-5
Engineered marble-like bovine fat tissue for cultured meat
Abstract
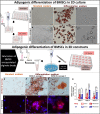
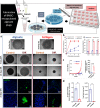
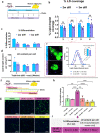
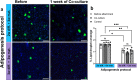
Cultured meat can provide a sustainable and more ethical alternative to conventional meat. Most of the research in this field has been focused on developing muscle tissue, as it is the main component of meat products, while very few studies address cultured fat tissue, an essential component in the human diet and determinant of meat quality, flavor, juiciness, and tenderness. Here, we engineered bovine fat tissue for cultured meat and incorporated it within engineered bovine muscle tissue. Mesenchymal stem cells (MSCs) were derived from bovine adipose tissue and exhibited the typical phenotypic profile of adipose-derived MSCs. MSC adipogenic differentiation and maturation within alginate-based three-dimensional constructs were optimized to yield a fat-rich edible engineered tissue. Subsequently, a marble-like construct, composed of engineered bovine adipose and muscle tissues, was fabricated, mimicking inter- and intra-muscular fat structures.
© 2022. The Author(s).
Conflict of interest statement
This research was sponsored by Aleph Farms. Prof. Shulamit Levenberg is the chief scientific advisor and Dr. Neta Lavon is the chief technology officer and vice president of R&D of Aleph Farms. The remaining authors declare no competing interests.
Figures

References
-
- Bhat ZF, Fayaz H. Prospectus of cultured meat—advancing meat alternatives. J. Food Sci. Technol. 2011;48:125–140.
-
- Post MJ. Cultured meat from stem cells: challenges and prospects. Meat Sci. 2012;92:297–301. - PubMed
-
- Zhang G, et al. Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci. Technol. 2020;97:443–450.
-
- Wood JD, et al. Effects of fatty acids on meat quality: a review. Meat Sci. 2004;66:21–32. - PubMed
-
- Ng S, Kurisawa M. Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomater. 2021;124:108–129. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

