S1P/S1PR1 signaling differentially regulates the allogeneic response of CD4 and CD8 T cells by modulating mitochondrial fission
- PMID: 36071219
- PMCID: PMC9622814
- DOI: 10.1038/s41423-022-00921-x
S1P/S1PR1 signaling differentially regulates the allogeneic response of CD4 and CD8 T cells by modulating mitochondrial fission
Abstract
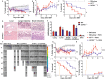
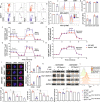
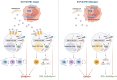
Graft-versus-host disease (GVHD) significantly contributes to patient morbidity and mortality after allogeneic hematopoietic cell transplantation (allo-HSCT). Sphingosine-1-phosphate (S1P) signaling is involved in the biogenetic processes of different immune cells. In the current study, we demonstrated that recipient sphingosine kinase 1 (Sphk1), but not Sphk2, was required for optimal S1PR1-dependent donor T-cell allogeneic responses by secreting S1P. Using genetic and pharmacologic approaches, we demonstrated that inhibition of Sphk1 or S1PR1 substantially attenuated acute GVHD (aGVHD) while retaining the graft-versus-leukemia (GVL) effect. At the cellular level, the Sphk1/S1P/S1PR1 pathway differentially modulated the alloreactivity of CD4+ and CD8+ T cells; it facilitated T-cell differentiation into Th1/Th17 cells but not Tregs and promoted CD4+ T-cell infiltration into GVHD target organs but was dispensable for the CTL activity of allogeneic CD8+ T cells. At the molecular level, the Sphk1/S1P/S1PR1 pathway augmented mitochondrial fission and increased mitochondrial mass in allogeneic CD4+ but not CD8+ T cells by activating the AMPK/AKT/mTOR/Drp1 pathway, providing a mechanistic basis for GVL maintenance when S1P signaling was inhibited. For translational purposes, we detected the regulatory efficacy of pharmacologic inhibitors of Sphk1 and S1PR1 in GVHD induced by human T cells in a xenograft model. Our study provides novel mechanistic insight into how the Sphk1/S1P/S1PR1 pathway modulates T-cell alloreactivity and validates Sphk1 or S1PR1 as a therapeutic target for the prevention of GVHD and leukemia relapse. This novel strategy may be readily translated into the clinic to benefit patients with hematologic malignancies and disorders.
Keywords: GVHD; GVL; S1P; S1PR; Sphk1; mitochondrial fission.
© 2022. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

