Low-dose spironolactone ameliorates adipose tissue inflammation and apoptosis in letrozole-induced PCOS rat model
- PMID: 36071485
- PMCID: PMC9454226
- DOI: 10.1186/s12902-022-01143-y
Low-dose spironolactone ameliorates adipose tissue inflammation and apoptosis in letrozole-induced PCOS rat model
Abstract
Background of study: Globally, many reproductive aged women are affected by polycystic ovarian syndrome (PCOS), which is a common endocrine and metabolic disorder that is linked with adipose dysfunction and chronic low-grade inflammation. Spironolactone (SPL), a mineralocorticoid receptor blocker has been documented as a metabolic modulator. However, its immunomodulatory effect in PCOS is unknown. Therefore, the present study hypothesized that SPL would ameliorate adipose dysfunction and inflammation in experimental PCOS animals.
Materials and methods: Female Wistar rats that were 8 weeks old were allocated into three groups. Group 1 received vehicle (distilled water; p.o.), group 2 received letrozole (1 mg/kg; p.o.) and group 3 received letrozole plus SPL (0.25 mg/kg, p.o.). The administration was performed once daily for 21 days.
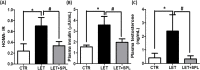
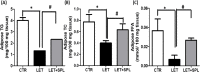
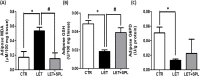
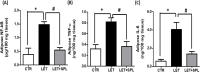
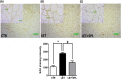
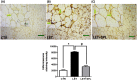
Results: The experimental PCOS animals showed insulin resistance, hyperinsulinemia and hyperandrogenism as well as oxidative stress and elevated inflammatory biomarkers (NF-kB/TNF-/IL-6) as well as a significant decrease in triglycerides, total cholesterol, free fatty acids, GSH and G6PD in the adipose tissue of PCOS animals. In addition, immunohistochemical assessment of adipose tissue showed significant expression of BAX and inflammasome, indicating apoptosis and inflammation compared to control animals. Nevertheless, administration of SPL attenuated these perturbations.
Conclusion: Altogether, the present study suggests that low-dose spironolactone confers protection against adipose dysfunction in experimental PCOS animals by attenuating inflammation, oxidative stress and cellular apoptosis.
Keywords: Adipose tissue; Inflammation; Oxidative stress; PCOS; Spironolactone.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kabel AM, Alghubayshi AY, Moharm FM. The impact of polycystic ovarian syndrome, a potential risk factor to endometrial cancer, on the quality of sleep. J Cancer Res Treat. 2016;4(6):96–98.
-
- Zafar U, Memon Z, Moin K, Agha S, Hassan JA, Zehra D. Prevalence of PCOS with associated symptoms and complications at tertiary care hospital of Karachi. J Adv Med Med Res. 2019;30(4):1–9. doi: 10.9734/jammr/2019/v30i430190. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

