Diagnostic performance of automated plasma amyloid-β assays combined with pre-analytical immunoprecipitation
- PMID: 36071505
- PMCID: PMC9450259
- DOI: 10.1186/s13195-022-01071-y
Diagnostic performance of automated plasma amyloid-β assays combined with pre-analytical immunoprecipitation
Abstract
Background: Measurements of the amyloid-β (Aβ) 42/40 ratio in blood plasma may support the early diagnosis of Alzheimer's disease and aid in the selection of suitable participants in clinical trials. Here, we compared the diagnostic performance of fully automated prototype plasma Aβ42/40 assays with and without pre-analytical sample workup by immunoprecipitation.
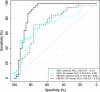
Methods: A pre-selected clinical sample comprising 42 subjects with normal and 38 subjects with low cerebrospinal fluid (CSF) Aβ42/40 ratios was studied. The plasma Aβ42/40 ratios were determined with fully automated prototype Elecsys® immunoassays (Roche Diagnostics GmbH, Penzberg, Germany) by direct measurements in EDTA plasma or after pre-analytical Aβ immunoprecipitation. The diagnostic performance for the detection of abnormal CSF Aβ42/40 was analyzed by receiver operating characteristic (ROC) analysis. In an additional post hoc analysis, a biomarker-supported clinical diagnosis was used as a second endpoint.
Results: Pre-analytical immunoprecipitation resulted in a significant increase in the area under the ROC curve (AUC) from 0.73 to 0.88 (p = 0.01547) for identifying subjects with abnormal CSF Aβ42/40. A similar improvement in the diagnostic performance by pre-analytical immunoprecipitation was also observed when a biomarker-supported clinical diagnosis was used as a second endpoint (AUC increase from 0.77 to 0.92, p = 0.01576).
Conclusions: Our preliminary observations indicate that pre-analytical Aβ immunoprecipitation can improve the diagnostic performance of plasma Aβ assays for detecting brain amyloid pathology. The findings may aid in the further development of blood-based immunoassays for Alzheimer's disease ultimately suitable for screening and routine use.
Keywords: Alzheimer’s disease; Biomarker assay; Immunoprecipitation; Plasma Amyloid-β 42/40; Pre-analytical sample workup.
© 2022. The Author(s).
Conflict of interest statement
JW has been an honorary speaker for Actelion, Amgen, Beeijing Yibai Science and Technology Ltd., Janssen Cilag, Med Update GmbH, Pfizer, Roche Pharma, and has been a member of the advisory boards of Abbott, Biogen, Boehringer Ingelheim, Lilly, MSD Sharp & Dohme, and Roche Pharma and receives fees as a consultant for Immungenetics and Roboscreen. JW holds the following patents: PCT/EP 2011 001724 and PCT/EP 2015 052945. SP is a full-time employee of Roche Diagnostics GmbH and holds shares of the company. EM and AJ are full-time employees of Roche Diagnostics GmbH. IL is shareholder and CEO of Roboscreen GmbH, and he and DO are full-time employees of Roboscreen GmbH. JS and ChB are employees of Microdiscovery GmbH, Berlin, Germany. HWK, JV, AJB, HE, BB, CR, CB, and NH declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

