Efficacy of three anti-malarial regimens for uncomplicated Plasmodium falciparum malaria in Cambodia, 2009-2011: a randomized controlled trial and brief review
- PMID: 36071520
- PMCID: PMC9450427
- DOI: 10.1186/s12936-022-04279-3
Efficacy of three anti-malarial regimens for uncomplicated Plasmodium falciparum malaria in Cambodia, 2009-2011: a randomized controlled trial and brief review
Abstract
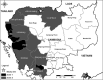
Background: Anti-malarial resistance remains an important public health challenge in Cambodia. The effectiveness of three therapies for uncomplicated falciparum malaria was evaluated in Oddar Meanchey province in Northern Cambodia from 2009 to 2011.
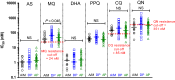
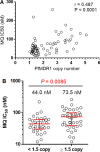
Methods: In this randomized, open-label, parallel group-controlled trial, 211 subjects at least 5 years old with uncomplicated falciparum malaria were treated with 3 days of directly observed therapy: 63 received artesunate-mefloquine (AS/MQ), 77 received dihydroartemisinin-piperaquine (DHA/PPQ), and 71 received atovaquone-proguanil (ATQ/PG). The subjects were followed for 42 days or until recurrent parasitaemia. Genotyping of msp1, msp2, and glurp among individual parasite isolates distinguished recrudescence from reinfection. Pfmdr1 copy number was measured by real-time PCR and half-maximal parasite inhibitory concentrations (IC50) were measured in vitro by 48-h isotopic hypoxanthine incorporation assay.
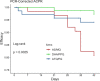
Results: The per-protocol PCR-adjusted efficacy (95% confidence interval) at 42 days was 80.6% (70.8-90.5%) for AS/MQ, 97.2% (93.3-100%) for DHA/PPQ, and 92.9% (86.1-99.6%) for ATQ/PG. On day 3, 57.9% remained parasitaemic in the AS/MQ and DHA/PPQ arms. At baseline, 46.9% had microscopic Plasmodium falciparum gametocytaemia. Both recurrences in the DHA/PPQ arm lost Pfmdr1 copy number amplification at recrudescence. All four recurrences in the ATQ/PG arm were wild-type for cytochrome bc1. One subject withdrew from the ATQ/PG arm due to drug allergy.
Conclusions: This study was conducted at the epicentre of substantial multi-drug resistance that emerged soon thereafter. Occurring early in the national transition from AS/MQ to DHA/PPQ, both DHA/PPQ and ATQ/PG had acceptable efficacy against uncomplicated falciparum malaria. However, efficacy of AS/MQ was only 80% with apparent mefloquine resistance based on elevated Pfmdr1 copy number and IC50. By 2009, there was already significant evidence of artemisinin resistance not previously reported at the Northern Cambodia-Thai border. This study suggests the basis for early development of significant DHA/PPQ failures within 3 years of introduction. Artemisinin resistance likely occurred on the Northern border concurrently with that reported along the Western border in Pailin. Trial registration This legacy trial was conducted prior to International Committee of Medical Journal Editors' requirements for preregistration on ClinicalTrials.gov. The full protocol has been provided.
Keywords: Antimalarial resistance; Cambodia; Randomized clinical trial; Therapeutic efficacy; Uncomplicated falciparum malaria.
© 2022. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures
Similar articles
-
Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia.Malar J. 2009 Jan 12;8:10. doi: 10.1186/1475-2875-8-10. Malar J. 2009. PMID: 19138388 Free PMC article.
-
Ex Vivo Drug Susceptibility Testing and Molecular Profiling of Clinical Plasmodium falciparum Isolates from Cambodia from 2008 to 2013 Suggest Emerging Piperaquine Resistance.Antimicrob Agents Chemother. 2015 Aug;59(8):4631-43. doi: 10.1128/AAC.00366-15. Epub 2015 May 26. Antimicrob Agents Chemother. 2015. PMID: 26014942 Free PMC article.
-
Ex vivo susceptibility of Plasmodium falciparum to antimalarial drugs in western, northern, and eastern Cambodia, 2011-2012: association with molecular markers.Antimicrob Agents Chemother. 2013 Nov;57(11):5277-83. doi: 10.1128/AAC.00687-13. Epub 2013 Aug 12. Antimicrob Agents Chemother. 2013. PMID: 23939897 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Atovaquone-proguanil for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD004529. doi: 10.1002/14651858.CD004529.pub3. Cochrane Database Syst Rev. 2021. PMID: 33459345 Free PMC article.
Cited by
-
Genetic surveillance of Plasmodium falciparum populations following treatment policy revisions in the Greater Mekong Subregion.Nat Commun. 2025 May 20;16(1):4689. doi: 10.1038/s41467-025-59946-1. Nat Commun. 2025. PMID: 40394107 Free PMC article.
-
In vitro activity of rhinacanthin analogues against drug resistant Plasmodium falciparum isolates from Northeast Thailand.Malar J. 2023 Mar 23;22(1):105. doi: 10.1186/s12936-023-04532-3. Malar J. 2023. PMID: 36959593 Free PMC article.
-
Evaluation of Dihydroartemisinin-Piperaquine Efficacy and Molecular Markers in Uncomplicated Falciparum Patients: A Study across Binh Phuoc and Dak Nong, Vietnam.Medicina (Kaunas). 2024 Jun 20;60(6):1013. doi: 10.3390/medicina60061013. Medicina (Kaunas). 2024. PMID: 38929629 Free PMC article.
-
Adherence to Anti-Malarial Treatment in Malaria Endemic Areas of Bangladesh.Pathogens. 2023 Nov 27;12(12):1392. doi: 10.3390/pathogens12121392. Pathogens. 2023. PMID: 38133277 Free PMC article.
References
-
- WHO . Artemisinin resistance and artemisinin-based combination therapy efficacy: status report. Geneva: World Health Organization; 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

