Interpreting alignment-free sequence comparison: what makes a score a good score?
- PMID: 36071721
- PMCID: PMC9442500
- DOI: 10.1093/nargab/lqac062
Interpreting alignment-free sequence comparison: what makes a score a good score?
Abstract
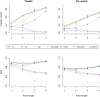
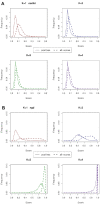
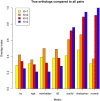
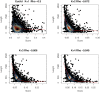
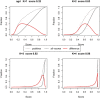
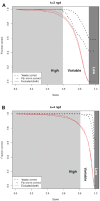
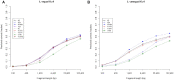
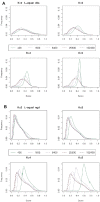
Alignment-free methods are alternatives to alignment-based methods when searching sequence data sets. The output from an alignment-free sequence comparison is a similarity score, the interpretation of which is not straightforward. We propose objective functions to interpret and calibrate outputs from alignment-free searches, noting that different objective functions are necessary for different biological contexts. This leads to advantages: visualising and comparing score distributions, including those from true positives, may be a relatively simple method to gain insight into the performance of different metrics. Using an empirical approach with both DNA and protein sequences, we characterise different similarity score distributions generated under different parameters. In particular, we demonstrate how sequence length can affect the scores. We show that scores of true positive sequence pairs may correlate significantly with their mean length; and even if the correlation is weak, the relative difference in length of the sequence pair may significantly reduce the effectiveness of alignment-free metrics. Importantly, we show how objective functions can be used with test data to accurately estimate the probability of true positives. This can significantly increase the utility of alignment-free approaches. Finally, we have developed a general-purpose software tool called KAST for use in high-throughput workflows on Linux clusters.
© The Author(s) 2022. Published by Oxford University Press on behalf of NAR Genomics and Bioinformatics.
Figures



References
-
- Needleman S.B., Wunsch C.D.. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 1970; 48:443–453. - PubMed
-
- Smith T.F., Waterman M.S.. Identification of common molecular subsequences. J. Mol. Biol. 1981; 147:195–197. - PubMed
-
- Vinga S., Almeida J.. Alignment-free sequence comparison—a review. Bioinformatics. 2003; 19:513–523. - PubMed
LinkOut - more resources
Full Text Sources

