The Use of Alloderm® Coverage to Reinforce Tissues in Two-Stage Tissue Expansion Placement in the Subcutaneous (Prepectoral) Plane: A Prospective Pilot Study
- PMID: 36072166
- PMCID: PMC9440738
- DOI: 10.7759/cureus.27680
The Use of Alloderm® Coverage to Reinforce Tissues in Two-Stage Tissue Expansion Placement in the Subcutaneous (Prepectoral) Plane: A Prospective Pilot Study
Abstract
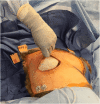
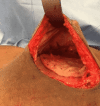
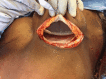
Purpose: Two-stage tissue expander (TE) to implant breast reconstruction is commonly performed by plastic surgeons. Prepectoral implant placement with acellular dermal matrix (ADM, e.g., AlloDerm®) reinforcement is evidenced by minimal postoperative pain. However, the same is not known for TE-based reconstruction. We performed this study to explore the use of complete AlloDerm® reinforcement of breast pocket tissues in women undergoing unilateral or bilateral mastectomies followed by immediate, two-stage tissue expansion in the prepectoral plane.
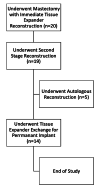
Methods: Patients (n = 20) aged 18-75 years were followed prospectively from their preoperative consult to 60 days post-TE insertion. The pain visual analog scale (VAS), Patient Pain Assessment Questionnaire, Subjective Pain Survey, Brief Pain Inventory-Short Form (BPI-SF), postoperative nausea and vomiting (PONV) survey, BREAST-Q Reconstruction Module, and short-form 36 (SF-36) questionnaires were administered. Demographic, intraoperative, and 30- and 60-day complications data were abstracted from medical records. After TE-to-implant exchange, patients were followed until 60 days postoperatively to assess for complications.
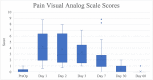
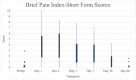
Results: Pain VAS and BPI-SF pain interference scores returned to preoperative values by 30 days post-TE insertion. Static and moving pain scores from the Patient Pain Assessment Questionnaire returned to preoperative baseline values by day 60. The mean subjective pain score was 3.0 (0.5 standard deviation) with seven patients scoring outside the standard deviation; none of these seven patients had a history of anxiety or depression. Median PONV scores remained at 0 from postoperative day 0 to day 7. Patient-reported opioid use dropped from 89.5% to 10.5% by postoperative day 30.
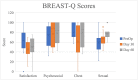
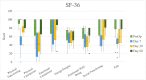
Breast-q: Sexual well-being scores significantly increased from preoperative baseline to day 60 post-TE insertion. Changes in SF-36 physical functioning, physician limitations, emotional well-being, social functioning, and pain scores were significantly different from preoperative baseline to day 60 post-TE insertion. Five participants had complications within 60 days post-TE insertion. One participant experienced a complication within 60 days after TE-to-implant exchange.
Conclusions: We describe pain scores, opioid usage, patient-reported outcomes data, and complication profiles of 20 consecutive patients undergoing mastectomy followed by immediate, two-stage tissue expansion in the prepectoral plane. We hope this study serves as a baseline for future research.
Keywords: adm; breast-q questionnaire; pain on vas; prepectoral breast reconstruction; sf-36 questionnaire; tissue expander.
Copyright © 2022, Tiongco et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Comparison of clinical outcomes and patient satisfaction in immediate single-stage versus two-stage implant-based breast reconstruction. Susarla SM, Ganske I, Helliwell L, Morris D, Eriksson E, Chun YS. Plast Reconstr Surg. 2015;135:1–8. - PubMed
-
- Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Sbitany H, Sandeen SN, Amalfi AN, Davenport MS, Langstein HN. Plast Reconstr Surg. 2009;124:1735–1740. - PubMed
-
- Pain after breast surgery: a survey of 282 women. Wallace MS, Wallace AM, Lee J, Dobke MK. Pain. 1996;66:195–205. - PubMed
LinkOut - more resources
Full Text Sources
