Trimethylamine N-oxide promotes demyelination in spontaneous hypertension rats through enhancing pyroptosis of oligodendrocytes
- PMID: 36072486
- PMCID: PMC9441869
- DOI: 10.3389/fnagi.2022.963876
Trimethylamine N-oxide promotes demyelination in spontaneous hypertension rats through enhancing pyroptosis of oligodendrocytes
Abstract
Background: Hypertension is a leading risk factor for cerebral small vessel disease (CSVD), a brain microvessels dysfunction accompanied by white matter lesions (WML). Trimethylamine N-oxide (TMAO), a metabolite of intestinal flora, is correlated with cardiovascular and aging diseases. Here, we explored the effect of TMAO on the demyelination of WML.
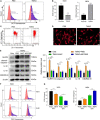
Methods: Spontaneous hypertension rats (SHRs) and primary oligodendrocytes were used to explore the effect of TMAO on demyelination in vivo and in vitro. T2-weighted magnetic resonance imaging (MRI) was applied to characterize the white matter hyperintensities (WMH) in rats. TMAO level was evaluated using LC-MS/MS assay. The histopathological changes of corpus callosum were measured by hematoxylin-eosin and luxol fast blue staining. And the related markers were detected by IHC, IF and western blot assay. Mito Tracker Red probe, DCFH-DA assay, flow cytometry based on JC-1 staining and Annexin V-FITC/PI double staining were conducted to evaluate the mitochondrial function, intracellular ROS levels and cell apoptosis.
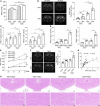
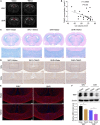
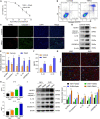
Results: SHRs exhibited stronger WMH signals and a higher TMAO level than age-matched normotensive Wistar-kyoto rats (WKY). The corpus callosum region of SHR showed decreased volumes and enhanced demyelination when treated with TMAO. Furthermore, TMAO significantly elevated ROS production and induced NLRP3 inflammasome and impairment of mitochondrial function of oligodendrocytes. More importantly, TMAO enhanced the pyroptosis-related inflammatory death of oligodendrocytes.
Conclusion: TMAO could cross the blood-brain barrier (BBB) and promote oligodendrocytes pyroptosis via ROS/NLRP3 inflammasome signaling and mitochondrial dysfunction to promote demyelination, revealing a new diagnostic marker for WML under hypertension.
Keywords: demyelination; hypertension; pyroptosis; trimethylamine N-oxide; white matter lesions.
Copyright © 2022 Ji, Tian, Niu, Yao and Qu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Brunt V. E., LaRocca T. J., Bazzoni A. E., Sapinsley Z. J., Miyamoto-Ditmon J., Gioscia-Ryan R. A., et al. (2021). “The gut microbiome-derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging.”. Geroscience 43 377–94. 10.1007/s11357-020-00257-2 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

