Coexistence of Pemphigus Vulgaris and Lichen Planus following COVID-19 Vaccination
- PMID: 36072649
- PMCID: PMC9441388
- DOI: 10.1155/2022/2324212
Coexistence of Pemphigus Vulgaris and Lichen Planus following COVID-19 Vaccination
Abstract
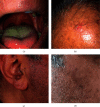
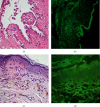
SARS-CoV-2 vaccines were approved without long-term monitoring due to emergent situation and might have several side effects. Herein, we describe the first case with development of both LP and PV following COVID-19 vaccination. Immunological alteration due to COVID-19 vaccination and its potential role in triggering autoimmune disorders were also dealt with.
Copyright © 2022 Zeinab Aryanian et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Kalantari Y., Sadeghzadeh-Bazargan A., Aryanian Z., Hatami P., Goodarzi A. First reported case of delayed-type hypersensitivity reaction to non-hyaluronic acid Polycaprolactone dermal filler following COVID-19 vaccination: a case report and a review of the literature. Clinical Case Reports . 2022;10(2) doi: 10.1002/ccr3.5343.e05343 - DOI - PMC - PubMed
-
- Hatami P., Aryanian Z., Nicknam Asl H., Goodarzi A. Mucocutaneous adverse effects following COVID-19 vaccination: a comprehensive review of the literature with a presentation of some cases from Iran. Iranian Journal of Dermatology . 2021;24(4):331–338. doi: 10.22034/IJD.2021.311094.1451. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

