Effect of Sufentanil Combined with Gabapentin on Acute Postoperative Pain in Patients Undergoing Intraspinal Tumor Resection: Study Protocol for a Randomized Controlled Trial
- PMID: 36072908
- PMCID: PMC9444033
- DOI: 10.2147/JPR.S374898
Effect of Sufentanil Combined with Gabapentin on Acute Postoperative Pain in Patients Undergoing Intraspinal Tumor Resection: Study Protocol for a Randomized Controlled Trial
Abstract
Purpose: Patients undergoing intraspinal tumor resection usually experience severe postoperative pain. Inadequate postoperative analgesia usually leads to severe postsurgical pain, which could cause patients to suffer from many other related complications. Recently, an increasing number of studies have found that gabapentin can relieve hyperalgesia, postoperative pain, and postoperative inflammation. However, there have been no reports on the use of gabapentin combined with sufentanil preoperatively for acute pain following intraspinal tumor resection.
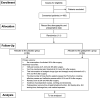
Study design and methods: This is a protocol for a randomized, placebo-controlled, and double-blinded trial. One-hundred and sixty-eight participants with chronic pain related to the intraspinal tumor will be randomized into the gabapentin and placebo groups in a 1:1 ratio. In the gabapentin group, patients will be given 300 mg gabapentin orally 36 h, 24 h, and 12 h before surgery; the placebo group will receive a placebo orally at the same time points preoperatively. To estimate the efficacy and safety endpoints, all the researchers and patients will be blinded until the completion of this study. The primary outcome will be the consumption of sufentanil within 48 h postoperatively. The secondary outcomes include the visual analog scale pain score and Von Frey mechanical pain threshold 36 h and 24 h before and 24 h and 48 h after surgery, the incidence of postoperative nausea, vomiting, and drowsiness, the length of hospital stay and medical expenses.
Discussion: This trial is to evaluate the efficacy and safety of gabapentin combined with sufentanil for postoperative analgesia in patients who complain of pain before intraspinal tumor resection. The findings will provide a new strategy for multimode perioperative analgesia management in these patients.
Keywords: gabapentin; intraspinal tumor resection; multimode perioperative analgesia; opiates.
© 2022 Zhang et al.
Conflict of interest statement
The authors declare no competing interests.
Similar articles
-
Effect of Intravenous Lidocaine on Postoperative Pain in Patients Undergoing Intraspinal Tumor Resection: Study Protocol for a Prospective Randomized Controlled Trial.J Pain Res. 2020 Jun 12;13:1401-1410. doi: 10.2147/JPR.S249359. eCollection 2020. J Pain Res. 2020. PMID: 32606906 Free PMC article.
-
Esketamine combined with pregabalin on acute postoperative pain in patients undergoing resection of spinal neoplasms: study protocol for a randomized controlled trial.Trials. 2023 Feb 25;24(1):144. doi: 10.1186/s13063-023-07178-3. Trials. 2023. PMID: 36841794 Free PMC article.
-
Effect of Perioperative Gabapentin Use on Postsurgical Pain in Patients Undergoing Head and Neck Mucosal Surgery: A Randomized Clinical Trial.JAMA Otolaryngol Head Neck Surg. 2018 Nov 1;144(11):959-966. doi: 10.1001/jamaoto.2018.0282. JAMA Otolaryngol Head Neck Surg. 2018. PMID: 29710075 Free PMC article. Clinical Trial.
-
Perioperative intravenous ketamine for acute postoperative pain in adults.Cochrane Database Syst Rev. 2018 Dec 20;12(12):CD012033. doi: 10.1002/14651858.CD012033.pub4. Cochrane Database Syst Rev. 2018. PMID: 30570761 Free PMC article.
-
Perioperative gabapentin and post cesarean pain control: A systematic review and meta-analysis of randomized controlled trials.Eur J Obstet Gynecol Reprod Biol. 2019 Feb;233:98-106. doi: 10.1016/j.ejogrb.2018.11.026. Epub 2018 Dec 12. Eur J Obstet Gynecol Reprod Biol. 2019. PMID: 30583095
References
LinkOut - more resources
Full Text Sources