Termination of CD40L co-stimulation promotes human B cell differentiation into antibody-secreting cells
- PMID: 36073009
- PMCID: PMC9825913
- DOI: 10.1002/eji.202249972
Termination of CD40L co-stimulation promotes human B cell differentiation into antibody-secreting cells
Abstract
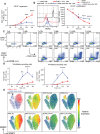
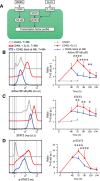
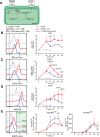
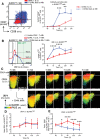
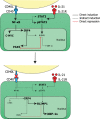
Human naïve B cells are notoriously difficult to differentiate into antibody-secreting cells (ASCs) in vitro while maintaining sufficient cell numbers to evaluate the differentiation process. B cells require T follicular helper (TFH ) cell-derived signals like CD40L and IL-21 during germinal center (GC) responses to undergo differentiation into ASCs. Cognate interactions between B and TFH cells are transient; after TFH contact, B cells cycle between GC light and dark zones where TFH contact is present and absent, respectively. Here, we elucidated that the efficacy of naïve B cells in ACS differentiation is dramatically enhanced by the release of CD40L stimulation. Multiparameter phospho-flow and transcription factor (TF)-flow cytometry revealed that termination of CD40L stimulation downmodulates NF-κB and STAT3 signaling. Furthermore, the termination of CD40 signaling downmodulates C-MYC, while promoting ASC TFs BLIMP1 and XBP-1s. Reduced levels of C-MYC in the differentiating B cells are later associated with crucial downmodulation of the B cell signature TF PAX5 specifically upon the termination of CD40 signaling, resulting in the differentiation of BLIMP1 high expressing cells into ASCs. The data presented here are the first steps to provide further insights how the transient nature of CD40 signaling is in fact needed for efficient human naïve B cell differentiation to ASCs.
Keywords: CD40L; IL-21; human B cells; phospho-signaling; transcriptional regulation.
© 2022 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

