Rapid and sensitive on-site genetic diagnostics of pest fruit flies using CRISPR-Cas12a
- PMID: 36073293
- PMCID: PMC10086973
- DOI: 10.1002/ps.7173
Rapid and sensitive on-site genetic diagnostics of pest fruit flies using CRISPR-Cas12a
Abstract
Background: Bactrocera zonata, a major fruit pest species, is gradually spreading west from its native habitat in East Asia. In recent years it has become a significant threat to the Mediterranean area, with the potential of invading Europe, the Americas, and Australia. To prevent it spreading, monitoring efforts in cultivation sites and border controls are carried out. Despite these efforts, and due to morphological similarities between B. zonata and other pests in relevant developmental stages, the monitoring process is challenging, time-consuming, and requires external assistance from professional laboratories. CRISPR-Cas12a genetic diagnostics has been rapidly developing in recent years and provides an efficient tool for the genetic identification of pathogens, viruses, and other genetic targets. Here we design a CRISPR-Cas12a detection assay that differentially detects two major pest species, B. zonata and Ceratitis capitata.
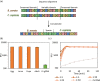
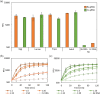
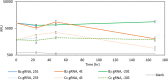
Results: We demonstrate the specificity and high sensitivity of this method. Identification of target pests was done using specific and universal primers on pooled samples, enabling differentiation of pests with high certainty. We also demonstrate reaction stability over time for future on-site applications.
Discussion: Our easy-to-use and affordable assay employs a simple DNA extraction technique together with isothermal amplification and Cas12a-based detection. This method is highly modular, and the presented target design method can be applied to a wide array of pests. This approach can be easily adapted to fit local threats and requires minimal training of operators in border controls and other relevant locations, reshaping pest control and making state-of-the-art technologies available worldwide, including in developing countries. © 2022 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Bactrocera zonata; CRISPR-Cas12a; Ceratitis capitata; RPA; genetic detection; pest-control.
© 2022 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Figures

References
-
- EPPO , Bactrocera zonata. EPPO Bull 35:371–373 (2005).
-
- Stonehouse JM, Mumford JD and Mustafa G, Economic losses to tephritid fruit flies (Diptera: Tephritidae) in Pakistan. Crop Prot 17:159–164 (1998).
-
- EPPO , PM 9/11 (1): Bactrocera zonata: procedure for official control. EPPO Bull 40:390–395 (2010).
-
- Delrio G and Cocco A, Peach fruit fly, Bactrocera zonata: a major threat for Mediterranean fruit crops? Acta Hortic 940:557–566 (2012).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

