Evaluating awareness, knowledge and practice of healthcare professionals following implementation of a revised pregnancy prevention programme for isotretinoin in Ireland: A multi-stakeholder cross-sectional study
- PMID: 36073295
- PMCID: PMC10092126
- DOI: 10.1002/pds.5538
Evaluating awareness, knowledge and practice of healthcare professionals following implementation of a revised pregnancy prevention programme for isotretinoin in Ireland: A multi-stakeholder cross-sectional study
Abstract
Purpose: In 2018, following an EU-wide safety review, a revised pregnancy prevention programme (PPP) was introduced for isotretinoin (Roaccutane®). This study aimed to examine awareness, knowledge, and experience implementing the revised isotretinoin PPP in clinical practice across three healthcare professional (HCP) groups in Ireland.
Methods: A cross-sectional study using anonymous online surveys among general practitioners (GPs), community pharmacists, and specialist consultants was undertaken. Descriptive analyses are presented.
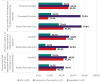
Results: Across all HCP groups there was high (≥87%) awareness that oral isotretinoin is contraindicated in women of childbearing potential (WCBP) unless the conditions of the PPP are fulfilled, but varying awareness among GPs (54.9%) and community pharmacists (45.9%) that exposure during pregnancy can cause both severe fetal malformations and spontaneous abortions. Implementation of the PPP in clinical practice varied across HCP groups. When initiating isotretinoin in WCBP, 66.7% of specialists and 40.8% of GPs indicated they had considered alternative treatment options, and 71.4% of specialists and 31.6% of GPs reported they first requested a pregnancy test. There was limited provision of the patient reminder card to WCBP, where 26.1% of community pharmacists provide this at each dispensing, while 47.6% of specialists and 11.8% of GPs ensured WCBP had a copy of the card when initiating treatment. Across all HCP groups, there was high (≥81.6%) awareness of the need for urgent consultation and immediate cessation of isotretinoin in the event of an unplanned or suspected pregnancy.
Conclusions: Reinforcement of the provision and utilisation of the isotretinoin patient reminder card may be required, and further targeted education on specific elements of the PPP should be considered for GPs and community pharmacists.
Keywords: isotretinoin; patient safety; pharmacoepidemiology; pharmacovigilance; pregnancy prevention program; teratogenicity.
© 2022 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures

References
-
- European Medicines Agency . Retinoid Article‐31 referral–PRAC assessment report 2018. Available from: https://www.ema.europa.eu/en/documents/referral/retinoid‐article‐31‐refe....
-
- Dai WS, LaBraico JM, et al. Epidemiology of isotretinoin exposure during pregnancy. J Am Acad Dermatol. 1992;26(4):599‐606. - PubMed
-
- Rosa F. Teratogenicity of isotretinoin. The Lancet. 1983;322(8348):513. - PubMed
-
- Stern RS, Rosa F, Baum C. Isotretinoin and pregnancy. J Am Acad Dermatol. 1984;10(5):851‐854. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

