ATIC-Associated De Novo Purine Synthesis Is Critically Involved in Proliferative Arterial Disease
- PMID: 36073366
- PMCID: PMC9643655
- DOI: 10.1161/CIRCULATIONAHA.121.058901
ATIC-Associated De Novo Purine Synthesis Is Critically Involved in Proliferative Arterial Disease
Abstract
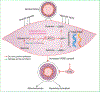
Background: Proliferation of vascular smooth muscle cells (VSMCs) is a hallmark of arterial diseases, especially in arterial restenosis after angioplasty or stent placement. VSMCs reprogram their metabolism to meet the increased requirements of lipids, proteins, and nucleotides for their proliferation. De novo purine synthesis is one of critical pathways for nucleotide synthesis. However, its role in proliferation of VSMCs in these arterial diseases has not been defined.
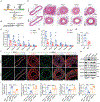
Methods: De novo purine synthesis in proliferative VSMCs was evaluated by liquid chromatography-tandem mass spectrometry. The expression of ATIC (5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/inosine monophosphate cyclohydrolase), the critical bifunctional enzyme in the last 2 steps of the de novo purine synthesis pathway, was assessed in VSMCs of proliferative arterial neointima. Global and VSMC-specific knockout of Atic mice were generated and used for examining the role of ATIC-associated purine metabolism in the formation of arterial neointima and atherosclerotic lesions.
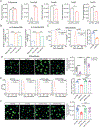
Results: In this study, we found that de novo purine synthesis was increased in proliferative VSMCs. Upregulated purine synthesis genes, including ATIC, were observed in the neointima of the injured vessels and atherosclerotic lesions both in mice and humans. Global or specific knockout of Atic in VSMCs inhibited cell proliferation, attenuating the arterial neointima in models of mouse atherosclerosis and arterial restenosis.
Conclusions: These results reveal that de novo purine synthesis plays an important role in VSMC proliferation in arterial disease. These findings suggest that targeting ATIC is a promising therapeutic approach to combat arterial diseases.
Keywords: ATIC; arterial diseases; atherosclerosis; de novo purine synthesis; proliferation; vascular smooth muscle cells.
Conflict of interest statement
Conflict of Interest Disclosures
None.
Figures





References
-
- Owens GK, Kumar MS and Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. - PubMed
-
- Schwartz SM, deBlois D and O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–65. - PubMed
-
- Dzau VJ, Braun-Dullaeus RC and Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–56. - PubMed
-
- Murray AW. The biological significance of purine salvage. Annu Rev Biochem. 1971;40:811–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

