Nonadherence by Serum Drug Analyses in Resistant Hypertension: 7-Year Follow-Up of Patients Considered Adherent by Directly Observed Therapy
- PMID: 36073648
- PMCID: PMC9683683
- DOI: 10.1161/JAHA.121.025879
Nonadherence by Serum Drug Analyses in Resistant Hypertension: 7-Year Follow-Up of Patients Considered Adherent by Directly Observed Therapy
Abstract
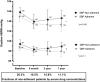
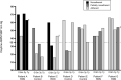
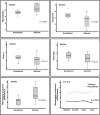
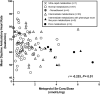
Background Measurement of serum concentrations of drugs is a novelty found useful in detecting poor drug adherence in patients taking ≥2 antihypertensive agents. Regarding patients with treatment-resistant hypertension, we previously based our assessment on directly observed therapy. The present study aimed to investigate whether serum drug measurements in patients with resistant hypertension offer additional information regarding drug adherence, beyond that of initial assessment with directly observed therapy. Methods and Results Nineteen patients assumed to have true treatment-resistant hypertension and adherence to antihypertensive drugs based on directly observed therapy were investigated repeatedly through 7 years. Serum concentrations of antihypertensive drugs were measured by ultra-high-performance liquid chromatography-tandem mass spectrometry from blood samples taken at baseline, 6-month, 3-year, and 7-year visits. Cytochrome P450 polymorphisms, self-reported adherence and beliefs about medicine were performed as supplement investigations. Seven patients (37%) were redefined as nonadherent based on their serum concentrations during follow-up. All patients reported high adherence to medications. Nonadherent patients expressed lower necessity and higher concerns regarding intake of antihypertensive medication (P=0.003). Cytochrome P450 polymorphisms affecting metabolism of antihypertensive drugs were found in 16 patients (84%), 21% were poor metabolizers, and none were ultra-rapid metabolizers. Six of 7 patients redefined as nonadherent had cytochrome P450 polymorphisms, however, not explaining the low serum drug concentrations measured in these patients. Conclusions Our data suggest that repeated measurements of serum concentrations of antihypertensive drugs revealed nonadherence in one-third of patients previously evaluated as adherent and treatment resistant by directly observed therapy, thereby improving the accuracy of adherence evaluation. Registration URL: https://www.clinicaltrials.gov; unique identifier: NCT01673516.
Keywords: antihypertensive drugs; blood pressure; directly observed therapy; nonadherence; resistant hypertension; serum drug concentrations.
Figures

Similar articles
-
Adherence to Antihypertensive Treatment and the Blood Pressure-Lowering Effects of Renal Denervation in the Renal Denervation for Hypertension (DENERHTN) Trial.Circulation. 2016 Sep 20;134(12):847-57. doi: 10.1161/CIRCULATIONAHA.116.022922. Epub 2016 Aug 30. Circulation. 2016. PMID: 27576780 Clinical Trial.
-
Routine urinary detection of antihypertensive drugs for systematic evaluation of adherence to treatment in hypertensive patients.J Hypertens. 2017 Sep;35(9):1891-1898. doi: 10.1097/HJH.0000000000001402. J Hypertens. 2017. PMID: 28505066
-
Nonadherence to antihypertensive medications is related to pill burden in apparent treatment-resistant hypertensive individuals.J Hypertens. 2020 Jun;38(6):1165-1173. doi: 10.1097/HJH.0000000000002398. J Hypertens. 2020. PMID: 32371807
-
Nonadherence in Hypertension: How to Develop and Implement Chemical Adherence Testing.Hypertension. 2022 Jan;79(1):12-23. doi: 10.1161/HYPERTENSIONAHA.121.17596. Epub 2021 Nov 5. Hypertension. 2022. PMID: 34739765 Review.
-
Is there any Hope for Monitoring Adherence in an Efficient and Feasible Way for Resistant Hypertension Diagnosis and Follow-Up?Curr Hypertens Rep. 2020 Oct 14;22(11):96. doi: 10.1007/s11906-020-01105-6. Curr Hypertens Rep. 2020. PMID: 33052474 Review.
Cited by
-
Effect of Therapeutic Drug Monitoring on Adherence and Blood Pressure: A Multicenter Randomized Clinical Trial.Am J Hypertens. 2024 Sep 16;37(10):826-836. doi: 10.1093/ajh/hpae059. Am J Hypertens. 2024. PMID: 38713475 Free PMC article. Clinical Trial.
-
Medical Telemonitoring for the Management of Hypertension in Older Patients in Japan.Int J Environ Res Public Health. 2023 Jan 26;20(3):2227. doi: 10.3390/ijerph20032227. Int J Environ Res Public Health. 2023. PMID: 36767594 Free PMC article. Review.
-
Therapeutic Drug Monitoring in Arterial Hypertension.J Pers Med. 2023 May 11;13(5):815. doi: 10.3390/jpm13050815. J Pers Med. 2023. PMID: 37240985 Free PMC article.
References
-
- Berra E, Azizi M, Capron A, Høieggen A, Rabbia F, Kjeldsen SE, Staessen JA, Wallemacq P, Persu A. Evaluation of adherence should become an integral part of assessment of patients with apparently treatment‐resistant hypertension. Hypertension. 2016;68:297–306. doi: 10.1161/HYPERTENSIONAHA.116.07464 - DOI - PubMed
-
- Hjørnholm UA, Larstorp ACK, Fadl Elmula FEM, Høieggen A, Andersen MH, Kjeldsen SE. Directly observed therapy in hypertension (DOT‐HTN). In: Burnier M, ed. Drug Adherence in Hypertension and Cardiovascular Protection. Springer Nature; 2018:57–85.
-
- Fadl Elmula FEM, Hoffmann P, Fossum E, Brekke M, Gjonnaess E, Hjornholm U, Kjær VN, Rostrup M, Kjeldsen SE, Os I, et al. Renal sympathetic denervation in patients with treatment‐resistant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension. 2013;62:526–532. doi: 10.1161/HYPERTENSIONAHA.113.01452 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

