Hydrophilic Chitosan Derivatives: Synthesis and Applications
- PMID: 36073726
- PMCID: PMC10092422
- DOI: 10.1002/chem.202202156
Hydrophilic Chitosan Derivatives: Synthesis and Applications
Abstract
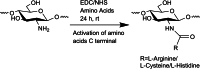
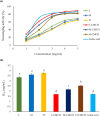
Polymer alternatives sourced from nature have attracted increasing attention for applications in medicine, cosmetics, agriculture, food, water purification, and more. Among them, chitosan is the most versatile due to its full biodegradability, exceptional biocompatibility, multipurpose bioactivity, and low toxicity. Although remarkable progress has been made in its synthetic modification by using C3/C6 secondary/primary hydroxy (-OH) and the C2 amino (-NH2 ) active sites, its solubility under physiological conditions remains limited and has hampered larger-scale adoption. This review summarizes different synthetic methods that increase chitosan's hydrophilicity and water solubility by using covalent modifications, namely amino acid addition, quaternary ammonium formation, phosphorylation, and carboxymethylation. We also review several applications for each type of substitution in fields such as cosmetics, medicine, agriculture, and water purification, and provide an outlook and perspective for future modifications and implementations.
Keywords: antibacterial; biopolymers; chitosan; hydrophilic substitution; nanoparticles.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







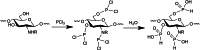














References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

