Regulation of pulmonary surfactant by the adhesion GPCR GPR116/ADGRF5 requires a tethered agonist-mediated activation mechanism
- PMID: 36073784
- PMCID: PMC9489211
- DOI: 10.7554/eLife.69061
Regulation of pulmonary surfactant by the adhesion GPCR GPR116/ADGRF5 requires a tethered agonist-mediated activation mechanism
Abstract
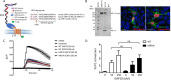
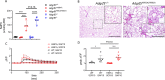
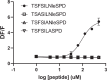
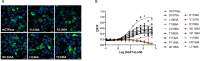
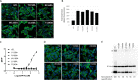
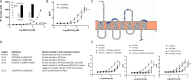
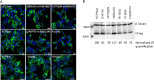
The mechanistic details of the tethered agonist mode of activation for the adhesion GPCR ADGRF5/GPR116 have not been completely deciphered. We set out to investigate the physiological importance of autocatalytic cleavage upstream of the agonistic peptide sequence, an event necessary for NTF displacement and subsequent receptor activation. To examine this hypothesis, we characterized tethered agonist-mediated activation of GPR116 in vitro and in vivo. A knock-in mouse expressing a non-cleavable GPR116 mutant phenocopies the pulmonary phenotype of GPR116 knock-out mice, demonstrating that tethered agonist-mediated receptor activation is indispensable for function in vivo. Using site-directed mutagenesis and species-swapping approaches, we identified key conserved amino acids for GPR116 activation in the tethered agonist sequence and in extracellular loops 2/3 (ECL2/3). We further highlight residues in transmembrane 7 (TM7) that mediate stronger signaling in mouse versus human GPR116 and recapitulate these findings in a model supporting tethered agonist:ECL2 interactions for GPR116 activation.
Keywords: adhesion GPCR ADGRF5; autocatalytic GPS cleavage; cell biology; human; modeling; mouse; pulmonary surfactant; tethered agonist activation.
© 2022, Bridges et al.
Conflict of interest statement
JB, KB, AF, RP, WM No competing interests declared, CS, BP, RB, SP, KS, ML is employed by and shareholder of Novartis Pharma AG, NR is employed by and shareholders of Novartis Pharma AG
Figures


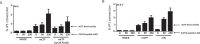



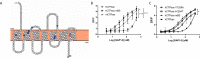
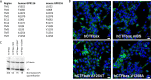


References
-
- Arimont M, van der Woude M, Leurs R, Vischer HF, de Graaf C, Nijmeijer S. Identification of key structural motifs involved in 7 transmembrane signaling of adhesion gpcrs. ACS Pharmacology & Translational Science. 2019;2:101–113. doi: 10.1021/acsptsci.8b00051. - DOI
-
- Barros-Álvarez X, Nwokonko RM, Vizurraga A, Matzov D, He F, Papasergi-Scott MM, Robertson MJ, Panova O, Yardeni EH, Seven AB, Kwarcinski FE, Su H, Peroto MC, Meyerowitz JG, Shalev-Benami M, Tall GG, Skiniotis G. The tethered peptide activation mechanism of adhesion gpcrs. Nature. 2022;604:757–762. doi: 10.1038/s41586-022-04575-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

