Syntrophic Acetate-Oxidizing Microbial Consortia Enriched from Full-Scale Mesophilic Food Waste Anaerobic Digesters Showing High Biodiversity and Functional Redundancy
- PMID: 36073802
- PMCID: PMC9600251
- DOI: 10.1128/msystems.00339-22
Syntrophic Acetate-Oxidizing Microbial Consortia Enriched from Full-Scale Mesophilic Food Waste Anaerobic Digesters Showing High Biodiversity and Functional Redundancy
Abstract
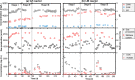
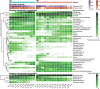
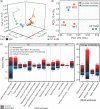
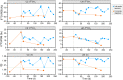
Syntrophic acetate oxidation (SAO) coupled with hydrogenotrophic methanogenesis (HM) plays a vital role in the anaerobic digestion of protein-rich feedstocks such as food wastes. However, current knowledge of the biodiversity and genetic potential of the involved microbial participants, especially syntrophic acetate-oxidizing bacteria (SAOB), is limited due to the low abundance of these microorganisms and challenges in their isolation. The intent of this study was to enrich and identify potential SAOB. Therefore, we conducted continuous acetate feeding under high ammonia concentrations using two separate inoculum consortia of microorganisms that originated from full-scale mesophilic food waste digesters, which lasted for more than 200 days. Using 16S rRNA gene amplicon and metagenomic analyses, we observed a convergence of the experimental microbial communities during the enrichment regarding taxonomic composition and metabolic functional composition. Stable carbon isotope analyses of biogas indicated that SAO-HM was the dominant methanogenic pathway during the enrichment process. The hydrogenotrophic methanogen Methanoculleus dominated the archaeal community. The enriched SAO community featured high biodiversity and metabolic functional redundancy. By analyzing the metagenome-assembled genomes, the known SAOB Syntrophaceticus schinkii and six uncultured populations were identified to have the genetic potential to perform SAO through the conventional reversed Wood-Ljungdahl pathway, while another six bacteria were found to encode the reversed Wood-Ljungdahl pathway combined with a glycine cleavage system as novel SAOB candidates. These results showed that the food waste anaerobic digesters harbor diverse SAOB and highlighted the importance of the glycine cleavage system for acetate oxidation. IMPORTANCE Syntrophic acetate oxidation to CO2 and H2, together with hydrogenotrophic methanogenesis, contributes to much of the carbon flux in the anaerobic digestion of organic wastes, especially at high ammonia concentrations. A deep understanding of the biodiversity, metabolic genetic potential, and ecology of the SAO community can help to improve biomethane production from wastes for clean energy production. Here, we enriched the SAO-HM functional guild obtained from full-scale food waste anaerobic digesters and recorded dynamic changes in community taxonomic composition and functional profiles. By reconstructing the metabolic pathways, diverse known and novel bacterial members were found to have SAO potential via the reversed Wood-Ljungdahl (WL) pathway alone, or via the reversed WL pathway with a glycine cleavage system (WLP-GCS), and those catalyzing WLP-GCS showed higher microbial abundance. This study revealed the biodiversity and metabolic functional redundancy of SAOB in full-scale anaerobic digester systems and provided inspiration for further genome-centric studies.
Keywords: anaerobic digestion; biodiversity; functional redundancy; metagenomic analysis; methanogenic pathway; syntrophic acetate oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fuchs W, Wang XM, Gabauer W, Ortner M, Li ZF. 2018. Tackling ammonia inhibition for efficient biogas production from chicken manure: status and technical trends in Europe and China. Renewable & Sustainable Energy Rev 97:186–199. doi: 10.1016/j.rser.2018.08.038. - DOI
-
- Bi S, Westerholm M, Hu W, Mahdy A, Dong T, Sun Y, Qiao W, Dong R. 2021. The metabolic performance and microbial communities of anaerobic digestion of chicken manure under stressed ammonia condition: a case study of a 10-year successful biogas plant. Renewable Energy 167:644–651. doi: 10.1016/j.renene.2020.11.133. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

