RcgA and RcgR, Two Novel Proteins Involved in the Conjugative Transfer of Rhizobial Plasmids
- PMID: 36073816
- PMCID: PMC9601222
- DOI: 10.1128/mbio.01949-22
RcgA and RcgR, Two Novel Proteins Involved in the Conjugative Transfer of Rhizobial Plasmids
Abstract
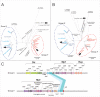
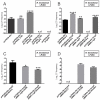
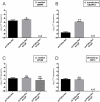
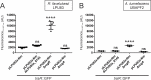
Rhizobia are Gram-negative bacteria that are able to establish a nitrogen-fixing symbiotic interaction with leguminous plants. Rhizobia genomes usually harbor several plasmids which can be transferred to other organisms by conjugation. Two main mechanisms of the regulation of rhizobial plasmid transfer have been described: quorum sensing (QS) and the rctA/rctB system. Nevertheless, new genes and molecules that modulate conjugative transfer have recently been described, demonstrating that new actors can tightly regulate the process. In this work, by means of bioinformatics tools and molecular biology approaches, two hypothetical genes are identified as playing key roles in conjugative transfer. These genes are located between conjugative genes of plasmid pRfaLPU83a from Rhizobium favelukesii LPU83, a plasmid that shows a conjugative transfer behavior depending on the genomic background. One of the two mentioned genes, rcgA, is essential for conjugation, while the other, rcgR, acts as an inhibitor of the process. In addition to introducing this new regulatory system, we show evidence of the functions of these genes in different genomic backgrounds and confirm that homologous proteins from non-closely related organisms have the same functions. These findings set up the basis for a new regulatory circuit of the conjugative transfer of plasmids. IMPORTANCE Extrachromosomal DNA elements, such as plasmids, allow for the adaptation of bacteria to new environments by conferring new determinants. Via conjugation, plasmids can be transferred between members of the same bacterial species, different species, or even to organisms belonging to a different kingdom. Knowledge about the regulatory systems of plasmid conjugative transfer is key in understanding the dynamics of their dissemination in the environment. As the increasing availability of genomes raises the number of predicted proteins with unknown functions, deeper experimental procedures help to elucidate the roles of these determinants. In this work, two uncharacterized proteins that constitute a new regulatory circuit with a key role in the conjugative transfer of rhizobial plasmids were discovered.
Keywords: Rhizobia; Rhizobium; conjugation; gene regulation; plasmid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Plasmid transfer systems in the rhizobia.Can J Microbiol. 2009 Aug;55(8):917-27. doi: 10.1139/w09-056. Can J Microbiol. 2009. PMID: 19898531 Review.
-
Insight into the structure, function and conjugative transfer of pLPU83a, an accessory plasmid of Rhizobium favelukesii LPU83.Plasmid. 2019 May;103:9-16. doi: 10.1016/j.plasmid.2019.03.004. Epub 2019 Mar 27. Plasmid. 2019. PMID: 30928704
-
Plasmid pSfr64a and the symbiotic plasmid pSfr64b of Sinorhizobium fredii GR64 control each other's conjugative transfer through quorum-sensing elements.Plasmid. 2019 Nov;106:102443. doi: 10.1016/j.plasmid.2019.102443. Epub 2019 Nov 2. Plasmid. 2019. PMID: 31689451
-
Rhizobial plasmid pLPU83a is able to switch between different transfer machineries depending on its genomic background.FEMS Microbiol Ecol. 2014 Jun;88(3):565-78. doi: 10.1111/1574-6941.12325. Epub 2014 Apr 7. FEMS Microbiol Ecol. 2014. PMID: 24646299
-
Regulation of conjugative transfer of plasmids and integrative conjugative elements.Plasmid. 2017 May;91:82-89. doi: 10.1016/j.plasmid.2017.04.002. Epub 2017 Apr 22. Plasmid. 2017. PMID: 28438469 Review.
Cited by
-
A plasmid-encoded inactive toxin-antitoxin system MtvT/MtvA regulates plasmid conjugative transfer and bacterial virulence in Pseudomonas aeruginosa.Nucleic Acids Res. 2025 Feb 8;53(4):gkaf075. doi: 10.1093/nar/gkaf075. Nucleic Acids Res. 2025. PMID: 39950345 Free PMC article.
-
Prevalence and Genomic Characteristics of mcr-Positive Escherichia coli Strains Isolated from Humans, Pigs, and Foods in China.Microbiol Spectr. 2023 Jun 15;11(3):e0456922. doi: 10.1128/spectrum.04569-22. Epub 2023 Apr 12. Microbiol Spectr. 2023. PMID: 37042751 Free PMC article.
-
Closed genomes of commercial inoculant rhizobia provide a blueprint for management of legume inoculation.Appl Environ Microbiol. 2025 Feb 19;91(2):e0221324. doi: 10.1128/aem.02213-24. Epub 2025 Jan 10. Appl Environ Microbiol. 2025. PMID: 39791879 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

