Transcript Engineered Extracellular Vesicles Alleviate Alloreactive Dynamics in Renal Transplantation
- PMID: 36073846
- PMCID: PMC9631077
- DOI: 10.1002/advs.202202633
Transcript Engineered Extracellular Vesicles Alleviate Alloreactive Dynamics in Renal Transplantation
Abstract
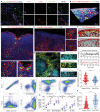
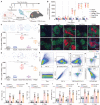
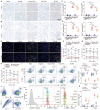
Direct contact of membrane molecules and cytokine interactions orchestrate immune homeostasis. However, overcoming the threshold of distance and velocity barriers, and achieving adhesion mediated immune interaction remain difficult. Here, inspired by the natural chemotaxis of regulatory T cells, multifunctionalized FOXP3 genetic engineered extracellular vesicles, termed Foe-TEVs, are designed, which display with adhesive molecules, regulatory cytokines, and coinhibitory contact molecules involving CTLA-4 and PD-1, by limited exogenous gene transduction. Foe-TEVs effectively adhere to the tubular, endothelial, and glomerular regions of allogeneic injury in the renal allograft, mitigating cell death in situ and chronic fibrosis transition. Remarkably, transcript engineering reverses the tracking velocity of vesicles to a retained phenotype and enhanced arrest coefficient by a factor of 2.16, directly interacting and attenuating excessive allosensitization kinetics in adaptive lymphoid organs. In murine allogeneic transplantation, immune adhesive Foe-TEVs alleviate pathological responses, restore renal function with well ordered ultrastructure and improved glomerular filtration rate, and prolong the survival period of the recipient from 30.16 to 92.81 days, demonstrating that the delivery of extracellular vesicles, genetically engineered for immune adhesive, is a promising strategy for the treatment of graft rejection.
Keywords: allograft rejection; genetic engineering; immune adhesion; renal transplantation.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- a) Doberer K., Duerr M., Halloran P. F., Eskandary F., Budde K., Regele H., Reeve J., Borski A., Kozakowski N., Reindl‐Schwaighofer R., Waiser J., Lachmann N., Schranz S., Firbas C., Muhlbacher J., Gelbenegger G., Perkmann T., Wahrmann M., Kainz A., Ristl R., Halleck F., Bond G., Chong E., Jilma B., Bohmig G. A., J. Am. Soc. Nephrol. 2021, 32, 708; - PMC - PubMed
- b) Lu X., Ru Y., Chu C., Lv Y., Gao Y., Jia Z., Huang Y., Zhang Y., Zhao S., Theranostics 2020, 10, 8446. - PMC - PubMed
-
- Belardi B., Son S., Felce J. H., Dustin M. L., Fletcher D. A., Nat. Rev. Mol. Cell Biol. 2020, 21, 750. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
