Transmissible Gastroenteritis Virus Infection Promotes the Self-Renewal of Porcine Intestinal Stem Cells via Wnt/β-Catenin Pathway
- PMID: 36073923
- PMCID: PMC9517692
- DOI: 10.1128/jvi.00962-22
Transmissible Gastroenteritis Virus Infection Promotes the Self-Renewal of Porcine Intestinal Stem Cells via Wnt/β-Catenin Pathway
Abstract
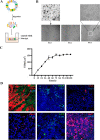
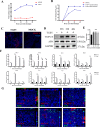
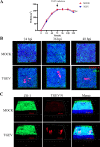
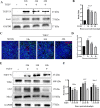
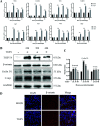
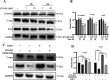
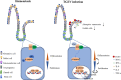
Intestinal stem cells (ISCs) play an important role in tissue repair after injury. A recent report delineates the effect of transmissible gastroenteritis virus (TGEV) infection on the small intestine of recovered pigs. However, the mechanism behind the epithelium regeneration upon TGEV infection remains unclear. To address this, we established a TGEV infection model based on the porcine intestinal organoid monolayer. The results illustrated that the porcine intestinal organoid monolayer was susceptible to TGEV. In addition, the TGEV infection initiated the interferon and inflammatory responses following the loss of absorptive enterocytes and goblet cells. However, TGEV infection did not disturb epithelial integrity but induced the proliferation of ISCs. Furthermore, TGEV infection activated the Wnt/β-catenin pathway by upregulating the accumulation and nuclear translocation of β-catenin, as well as promoting the expression of Wnt target genes, such as C-myc, Cyclin D1, Mmp7, Lgr5, and Sox9, which were associated with the self-renewal of ISCs. Collectively, these data demonstrated that the TGEV infection activated the Wnt/β-catenin pathway to promote the self-renewal of ISCs and resulted in intestinal epithelium regeneration. IMPORTANCE The intestinal epithelium is a physical barrier to enteric viruses and commensal bacteria. It plays an essential role in maintaining the balance between the host and intestinal microenvironment. In addition, intestinal stem cells (ISCs) are responsible for tissue repair after injury. Therefore, prompt self-renewal of intestinal epithelium will facilitate the rebuilding of the physical barrier and maintain gut health. In the manuscript, we found that the transmissible gastroenteritis virus (TGEV) infection did not disturb epithelial integrity but induced the proliferation of ISCs and facilitated epithelium regeneration. Detailed mechanism investigations revealed that the TGEV infection activated the Wnt/β-catenin pathway to promote the self-renewal of ISCs and resulted in intestinal epithelium regeneration. These findings will contribute to understanding the mechanism of intestinal epithelial regeneration and reparation upon viral infection.
Keywords: TGEV; Wnt/β-catenin pathway; epithelium regeneration; intestinal organoid monolayer; intestinal stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Abortive PDCoV infection triggers Wnt/β-catenin pathway activation, enhancing intestinal stem cell self-renewal and promoting chicken resistance.J Virol. 2025 Apr 15;99(4):e0013725. doi: 10.1128/jvi.00137-25. Epub 2025 Mar 26. J Virol. 2025. PMID: 40135895 Free PMC article.
-
Transmissible gastroenteritis virus targets Paneth cells to inhibit the self-renewal and differentiation of Lgr5 intestinal stem cells via Notch signaling.Cell Death Dis. 2020 Jan 20;11(1):40. doi: 10.1038/s41419-020-2233-6. Cell Death Dis. 2020. PMID: 31959773 Free PMC article.
-
Next-Generation Porcine Intestinal Organoids: an Apical-Out Organoid Model for Swine Enteric Virus Infection and Immune Response Investigations.J Virol. 2020 Oct 14;94(21):e01006-20. doi: 10.1128/JVI.01006-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32796075 Free PMC article.
-
Plasticity of Intestinal Epithelium: Stem Cell Niches and Regulatory Signals.Int J Mol Sci. 2020 Dec 31;22(1):357. doi: 10.3390/ijms22010357. Int J Mol Sci. 2020. PMID: 33396437 Free PMC article. Review.
-
Molecular regulation mechanism of intestinal stem cells in mucosal injury and repair in ulcerative colitis.World J Gastroenterol. 2023 Apr 28;29(16):2380-2396. doi: 10.3748/wjg.v29.i16.2380. World J Gastroenterol. 2023. PMID: 37179583 Free PMC article. Review.
Cited by
-
Potassium molybdate blocks APN-dependent coronavirus entry by degrading receptor via PIK3C3-mediated autophagy.J Virol. 2025 Jan 31;99(1):e0144924. doi: 10.1128/jvi.01449-24. Epub 2024 Dec 6. J Virol. 2025. PMID: 39641621 Free PMC article.
-
Bovine Enteroids as an In Vitro Model for Infection with Bovine Coronavirus.Viruses. 2023 Feb 27;15(3):635. doi: 10.3390/v15030635. Viruses. 2023. PMID: 36992344 Free PMC article.
-
Lactobacillus salivarius metabolite succinate enhances chicken intestinal stem cell activities via the SUCNR1-mitochondria axis.Poult Sci. 2025 Feb;104(2):104754. doi: 10.1016/j.psj.2024.104754. Epub 2024 Dec 31. Poult Sci. 2025. PMID: 39764876 Free PMC article.
-
A Strainer-Based Platform for the Collection and Immunolabeling of Porcine Epidemic Diarrhea Virus-Infected Porcine Intestinal Organoid.Int J Mol Sci. 2023 Oct 27;24(21):15671. doi: 10.3390/ijms242115671. Int J Mol Sci. 2023. PMID: 37958655 Free PMC article.
-
Wuzhishan miniature pig-derived intestinal 2D monolayer organoids to investigate the enteric coronavirus infection.Front Vet Sci. 2024 Sep 25;11:1457719. doi: 10.3389/fvets.2024.1457719. eCollection 2024. Front Vet Sci. 2024. PMID: 39386251 Free PMC article.
References
-
- Zhao X, Bai X, Guan L, Li J, Song X, Ma X, Guo J, Zhang Z, Du Q, Huang Y, Tong D. 2018. microRNA-4331 promotes transmissible gastroenteritis virus (TGEV)-induced mitochondrial damage via targeting RB1, upregulating interleukin-1 receptor accessory protein (IL1RAP), and activating p38 MAPK pathway in vitro. Mol Cell Proteomics 17:190–204. 10.1074/mcp.RA117.000432. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

