Characterization of MroQ-Dependent Maturation and Export of the Staphylococcus aureus Accessory Gene Regulatory System Autoinducing Peptide
- PMID: 36073934
- PMCID: PMC9584314
- DOI: 10.1128/iai.00263-22
Characterization of MroQ-Dependent Maturation and Export of the Staphylococcus aureus Accessory Gene Regulatory System Autoinducing Peptide
Abstract
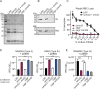
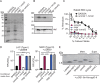
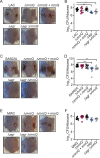
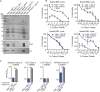
Gram-positive bacteria produce small autoinducing peptides (AIPs), which act to regulate expression of genes that promote adaptive traits, including virulence. The Gram-positive pathogen Staphylococcus aureus generates a cyclic AIP that controls expression of virulence factors via the accessory gene regulatory (Agr) system. S. aureus strains belong to one of four Agr groups (Agr-I, -II, -III, and -IV); each group harbors allelic variants of AgrD, the precursor of AIP. In a prior screen for S. aureus virulence factors, we identified MroQ, a putative peptidase. A ΔmroQ mutant closely resembled a Δagr mutant and had significant defects in AIP production in an Agr-I strain. Here, we show that expression of AgrD-I in a ΔmroQ mutant leads to accumulation of an AIP processing intermediate at the membrane that coincides with a loss of secreted mature AIP, indicating that MroQ promotes maturation of AgrD-I. MroQ is conserved in all Agr sequence variants, suggesting either identical function among all Agr types or activity specific to Agr-I strains. Our data indicate that MroQ is required for AIP maturation and activity in Agr-I, -II, and -IV strains irrespective of background. However, MroQ is not required for Agr-III activity despite an identifiable role in peptide maturation. Isogenic Δagr and Δagr ΔmroQ strains complemented with Agr-I to -IV validated the critical role of MroQ in the generation of active AIP-I, -II, and -IV but not AIP-III. These findings were reinforced by skin infection studies with mice. Our data substantiate the prevailing model that MroQ is a mediator of cyclic peptide maturation.
Keywords: Agr; MroQ; Staphylococcus aureus; peptidase; peptide; pheromone; quorum sensing; skin infection; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Determinants of maturation of the Staphylococcus aureus autoinducing peptide.J Bacteriol. 2024 Sep 19;206(9):e0019524. doi: 10.1128/jb.00195-24. Epub 2024 Aug 23. J Bacteriol. 2024. PMID: 39177535 Free PMC article.
-
Control of Staphylococcus aureus Quorum Sensing by a Membrane-Embedded Peptidase.Infect Immun. 2019 Apr 23;87(5):e00019-19. doi: 10.1128/IAI.00019-19. Print 2019 Mar. Infect Immun. 2019. PMID: 30833334 Free PMC article.
-
Reconstitution of the S. aureus agr quorum sensing pathway reveals a direct role for the integral membrane protease MroQ in pheromone biosynthesis.Proc Natl Acad Sci U S A. 2022 Aug 16;119(33):e2202661119. doi: 10.1073/pnas.2202661119. Epub 2022 Aug 8. Proc Natl Acad Sci U S A. 2022. PMID: 35939668 Free PMC article.
-
Quorum-sensing, intra- and inter-species competition in the staphylococci.Microbiology (Reading). 2023 Aug;169(8):001381. doi: 10.1099/mic.0.001381. Microbiology (Reading). 2023. PMID: 37578829 Free PMC article. Review.
-
Microbial Primer: agr-mediated quorum sensing in Gram-positive pathogens.Microbiology (Reading). 2025 Jul;171(7):001590. doi: 10.1099/mic.0.001590. Microbiology (Reading). 2025. PMID: 40742629 Free PMC article. Review.
Cited by
-
Molecular Mechanisms of Virulence Regulation in Staphylococcus aureus: A Journey into Reconstitutive Biochemistry.Acc Chem Res. 2025 May 20;58(10):1657-1669. doi: 10.1021/acs.accounts.5c00117. Epub 2025 May 7. Acc Chem Res. 2025. PMID: 40331756
-
Molecular structural arrangement in quorum sensing and bacterial metabolic production.World J Microbiol Biotechnol. 2025 Feb 13;41(2):71. doi: 10.1007/s11274-025-04280-3. World J Microbiol Biotechnol. 2025. PMID: 39939401 Review.
-
Pathogenic role of the staphylococcal accessory gene regulator quorum sensing system in atopic dermatitis.Front Cell Infect Microbiol. 2023 Apr 14;13:1178650. doi: 10.3389/fcimb.2023.1178650. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37124047 Free PMC article. Review.
-
Virulence attributes of successful methicillin-resistant Staphylococcus aureus lineages.Clin Microbiol Rev. 2023 Dec 20;36(4):e0014822. doi: 10.1128/cmr.00148-22. Epub 2023 Nov 20. Clin Microbiol Rev. 2023. PMID: 37982596 Free PMC article. Review.
-
Determinants of maturation of the Staphylococcus aureus autoinducing peptide.J Bacteriol. 2024 Sep 19;206(9):e0019524. doi: 10.1128/jb.00195-24. Epub 2024 Aug 23. J Bacteriol. 2024. PMID: 39177535 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

