The Combination of Platelet Rich Plasma Gel, Human Umbilical Mesenchymal Stem Cells and Nanohydroxyapatite/polyamide 66 Promotes Angiogenesis and Bone Regeneration in Large Bone Defect
- PMID: 36074328
- PMCID: PMC9679130
- DOI: 10.1007/s13770-022-00471-3
The Combination of Platelet Rich Plasma Gel, Human Umbilical Mesenchymal Stem Cells and Nanohydroxyapatite/polyamide 66 Promotes Angiogenesis and Bone Regeneration in Large Bone Defect
Abstract
Background: In the present study, a novel tissue engineering bone graft including platelet rich plasma gel (PRP gel), human umbilical mesenchymal stem cells (HUMSCs) and nanohydroxyapatite/polyamide 66 (nHA-PA66) was constructed. We explored whether the composite scaffolds could enhance the angiogenesis and bone repair capacity in rat femoral large bone defect (LBD). This study aimed to provide evidence for the clinical application of the composite scaffold in LBD treatment.
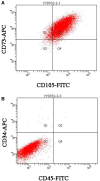
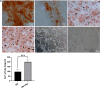
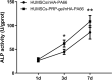
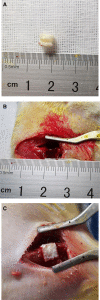
Methods: PRP was prepared, the platelets and growth factors were measured. HUMSCs were isolated and identified. the osteogenic capacity of PRP in vitro was measured. Then HUMSCs-PRP-gel/nHA-PA66 composite scaffolds were synthesized and observed. The proliferation and osteogenesis differentiation of HUMSCs on the composite scaffold was measured. The angiogenic capacity of PRP in vitro was measured by capillary-like tube formation assay. Finally, the angiogenesis and bone repair capacity of the composite scaffolds was measured in rat LBD.
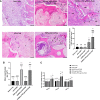
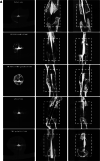
Results: PRP contained high level of platelets and growth factors after activation, and promoted osteogenic and angiogenic differentiation in vitro. The HUMSCs-PRP-gel/nHA-PA66 composite scaffold was porosity and promoted the proliferation and osteogenesis differentiation of HUMSCs. At 12th weeks, more micro-vessels and new bone were formed around the composite scaffolds compared with other groups, the defect was almost repaired.
Conclusion: Our study for the first time identified that the combination of PRP gel, HUMSCs and nHA-PA66 scaffold could significantly promote angiogenesis and bone regeneration in rat LBD, which may have implications for its further application in clinical LBD treatment.
Keywords: Angiogenesis; Large bone defect; Nanohydroxyapatite/polyamide 66; Osteogenesis; Platelet rich plasma.
© 2022. Korean Tissue Engineering and Regenerative Medicine Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
The Masquelet induced membrane technique with PRP-FG-nHA/PA66 scaffold can heal a rat large femoral bone defect.BMC Musculoskelet Disord. 2024 Jun 8;25(1):455. doi: 10.1186/s12891-024-07567-y. BMC Musculoskelet Disord. 2024. PMID: 38851675 Free PMC article.
-
The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects.Stem Cell Res Ther. 2017 May 25;8(1):122. doi: 10.1186/s13287-017-0574-6. Stem Cell Res Ther. 2017. PMID: 28545565 Free PMC article.
-
Nano-hydroxy apatite/chitosan/gelatin scaffolds enriched by a combination of platelet-rich plasma and fibrin glue enhance proliferation and differentiation of seeded human dental pulp stem cells.Biomed Pharmacother. 2019 Jan;109:1924-1931. doi: 10.1016/j.biopha.2018.11.072. Epub 2018 Nov 26. Biomed Pharmacother. 2019. PMID: 30551447
-
Umbilical cord mesenchymal stem cells combined with autologous platelet-rich plasma for lower extremity venous ulcers: A case report and literature review.Medicine (Baltimore). 2024 Nov 8;103(45):e40433. doi: 10.1097/MD.0000000000040433. Medicine (Baltimore). 2024. PMID: 39533589 Free PMC article. Review.
-
Platelet-rich plasma in orthopedics: Bridging innovation and clinical applications for bone repair.J Orthop Surg (Hong Kong). 2024 Jan-Apr;32(1):10225536231224952. doi: 10.1177/10225536231224952. J Orthop Surg (Hong Kong). 2024. PMID: 38217531 Review.
Cited by
-
Define of Optimal Addition Period of Osteogenic Peptide to Accelerate the Osteogenic Differentiation of Human Pluripotent Stem Cells.Tissue Eng Regen Med. 2024 Feb;21(2):291-308. doi: 10.1007/s13770-023-00597-y. Epub 2023 Oct 30. Tissue Eng Regen Med. 2024. PMID: 37903982 Free PMC article.
-
Engineered three-dimensional bioactive scaffold for enhanced bone regeneration through modulating transplanted adipose derived mesenchymal stem cell and stimulating angiogenesis.Front Bioeng Biotechnol. 2024 Jan 26;12:1342590. doi: 10.3389/fbioe.2024.1342590. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38344289 Free PMC article.
-
The Masquelet induced membrane technique with PRP-FG-nHA/PA66 scaffold can heal a rat large femoral bone defect.BMC Musculoskelet Disord. 2024 Jun 8;25(1):455. doi: 10.1186/s12891-024-07567-y. BMC Musculoskelet Disord. 2024. PMID: 38851675 Free PMC article.
-
A New Anatomical Nano Interbody Fusion Device for Elderly Patients Undergoing Anterior Discectomy and Interbody Fusion.Mol Biotechnol. 2025 Apr 1. doi: 10.1007/s12033-025-01427-3. Online ahead of print. Mol Biotechnol. 2025. PMID: 40169476
-
Demineralized bone matrix combined with concentrated growth factors promotes intervertebral fusion in a novel rat extreme lateral interbody fusion model.J Orthop Surg Res. 2025 May 27;20(1):529. doi: 10.1186/s13018-025-05954-2. J Orthop Surg Res. 2025. PMID: 40426199 Free PMC article.
References
-
- Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42:S3–15. - PubMed
-
- Berner A, Reichert JC, Müller MB, Zellner J, Pfeifer C, Dienstknecht T, et al. Treatment of long bone defects and non-unions: from research to clinical practice. Cell Tissue Res. 2012;347:501–519. - PubMed
-
- Cui Y, Zhu T, Li A, Liu B, Cui Z, Qiao Y, et al. Porous particle-reinforced bioactive gelatin scaffold for large segmental bone defect repairing. ACS Appl Mater Interfaces. 2018;10:6956–6964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
