Maternal alloxan exposure induces damage in rat offspring lumbar vertebrae and protective role of arachidonic acid
- PMID: 36074671
- PMCID: PMC9593121
- DOI: 10.47162/RJME.63.1.08
Maternal alloxan exposure induces damage in rat offspring lumbar vertebrae and protective role of arachidonic acid
Abstract
Background: Vertebral abnormalities in offspring of diabetic mothers make major challenges worldwide and were not sufficiently studied before.
Aim: To investigate the effects of alloxan-induced diabetes on rats' lumbar vertebrae, and to assess the potential beneficial impact of arachidonic acid.
Materials and methods: Pregnant rats were randomly equally divided into four groups: control, alloxan-induced diabetes received alloxan injection 150 mg∕kg, alloxan + arachidonic acid group received arachidonic acid 10 μg∕animal then given alloxan injection, and arachidonic acid group received it, until offspring age of three weeks. Six male offspring from each group were included in this study at ages of newborn, three-week-old, two-month-old, and their body measurements were recorded. Lumbar vertebrae and pancreas specimens were examined by light microscopy, morphometry, transmission electron microscopy (TEM), and immunohistochemistry for insulin expression.
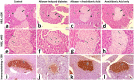
Results: In alloxan-induced diabetes newborn, three-week-old, and two-month-old rats, body measurements were significantly declined, histomorphometry of 6th lumbar vertebrae revealed disorganized chondrocytes, with vacuolated cytoplasm, empty lacunae, diminished matrix staining, with areas devoid of cells. TEM showed shrunken reserve and proliferative cells, with irregular nuclei, and damaged mitochondria. In contrast, alloxan + arachidonic acid group had cytoarchitecture of lumbar vertebrae that were like control group. Histomorphometry of pancreas in alloxan-induced diabetes group showed significant reduction in pancreatic islets number and surface area, damaged pancreatic islet cells appeared atrophied with apoptotic nuclei, and very weak insulin immunostaining. Whereas alloxan + arachidonic acid group displayed healthy features of pancreatic islets, which resembled control group, with strong insulin immunostaining.
Conclusions: Arachidonic acid mitigated alloxan-induced diabetes by its antidiabetic activity.
Conflict of interest statement
The authors declare that there is no conflict of interests.
Figures






References
-
- Bell R, Bailey K, Cresswell T, Hawthorne G, Critchley J, Lewis-Barned N, Northern Diabetic Pregnancy Survey Steering Group Trends in prevalence and outcomes of pregnancy in women with pre-existing type I and type II diabetes. BJOG. 2008;115(4):445–452. - PubMed
-
- Knight KM, Pressman EK, Hackney DN, Thornburg LL. Perinatal outcomes in type 2 diabetic patients compared with non-diabetic patients matched by body mass index. J Matern Fetal Neonatal Med. 2012;25(6):611–615. - PubMed
-
- Vinceti M, Malagoli C, Rothman KJ, Rodolfi R, Astolfi G, Calzolari E, Puccini A, Bertolotti M, Lunt M, Paterlini L, Martini M, Nicolini F. Risk of birth defects associated with maternal pregestational diabetes. Eur J Epidemiol. 2014;29(6):411–418. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

