Arbovirus-vector protein interactomics identifies Loquacious as a co-factor for dengue virus replication in Aedes mosquitoes
- PMID: 36074777
- PMCID: PMC9488832
- DOI: 10.1371/journal.ppat.1010329
Arbovirus-vector protein interactomics identifies Loquacious as a co-factor for dengue virus replication in Aedes mosquitoes
Abstract
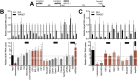
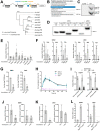
Efficient virus replication in Aedes vector mosquitoes is essential for the transmission of arboviral diseases such as dengue virus (DENV) in human populations. Like in vertebrates, virus-host protein-protein interactions are essential for viral replication and immune evasion in the mosquito vector. Here, 79 mosquito host proteins interacting with DENV non-structural proteins NS1 and NS5 were identified by label-free mass spectrometry, followed by a functional screening. We confirmed interactions with host factors previously observed in mammals, such as the oligosaccharyltransferase complex, and we identified protein-protein interactions that seem to be specific for mosquitoes. Among the interactors, the double-stranded RNA (dsRNA) binding protein Loquacious (Loqs), an RNA interference (RNAi) cofactor, was found to be essential for efficient replication of DENV and Zika virus (ZIKV) in mosquito cells. Loqs did not affect viral RNA stability or translation of a DENV replicon and its proviral activity was independent of its RNAi regulatory activity. Interestingly, Loqs colocalized with DENV dsRNA replication intermediates in infected cells and directly interacted with high affinity with DENV RNA in the 3' untranslated region in vitro (KD = 48-62 nM). Our study provides an interactome for DENV NS1 and NS5 and identifies Loqs as a key proviral host factor in mosquitoes. We propose that DENV hijacks a factor of the RNAi mechanism for replication of its own RNA.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Fischer D, Thomas SM, Neteler M, Tjaden NB, Beierkuhnlein C. Climatic suitability of Aedes albopictus in Europe referring to climate change projections: comparison of mechanistic and correlative niche modelling approaches. Eurosurveillance. 2014;19: 20696. doi: 10.2807/1560-7917.es2014.19.6.20696 - DOI - PubMed
-
- Aranda C, Martínez MJ, Montalvo T, Eritja R, Navero-Castillejos J, Herreros E, et al. Arbovirus surveillance: first dengue virus detection in local aedes albopictus mosquitoes in Europe, Catalonia, Spain, 2015. Eurosurveillance. 2018;23: e08347. doi: 10.2807/1560-7917.ES.2018.23.47.1700837 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

