Integrated biosensors for monitoring microphysiological systems
- PMID: 36074812
- PMCID: PMC9635816
- DOI: 10.1039/d2lc00262k
Integrated biosensors for monitoring microphysiological systems
Erratum in
-
Correction: Integrated biosensors for monitoring microphysiological systems.Lab Chip. 2024 Apr 16;24(8):2358-2359. doi: 10.1039/d4lc90026j. Lab Chip. 2024. PMID: 38501991 Free PMC article.
Abstract
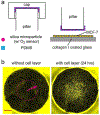
Microphysiological systems (MPSs), also known as organ-on-a-chip models, aim to recapitulate the functional components of human tissues or organs in vitro. Over the last decade, with the advances in biomaterials, 3D bioprinting, and microfluidics, numerous MPSs have emerged with applications to study diseased and healthy tissue models. Various organs have been modeled using MPS technology, such as the heart, liver, lung, and blood-brain barrier. An important aspect of in vitro modeling is the accurate phenotypical and functional characterization of the modeled organ. However, most conventional characterization methods are invasive and destructive and do not allow continuous monitoring of the cells in culture. On the other hand, microfluidic biosensors enable in-line, real-time sensing of target molecules with an excellent limit of detection and in a non-invasive manner, thereby effectively overcoming the limitation of the traditional techniques. Consequently, microfluidic biosensors have been increasingly integrated into MPSs and used for in-line target detection. This review discusses the state-of-the-art microfluidic biosensors by providing specific examples, detailing their main advantages in monitoring MPSs, and highlighting current developments in this field. Finally, we describe the remaining challenges and potential future developments to advance the current state-of-the-art in integrated microfluidic biosensors.
Conflict of interest statement
Conflicts of Interest:
The authors declare no conflict of interest.
Figures
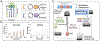





References
-
- Roth A and Berlin M-W, Science, 2021, 373, 1304–1306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

