Manganese Alkyl Carbonyl Complexes: From Iconic Stoichiometric Textbook Reactions to Catalytic Applications
- PMID: 36074912
- PMCID: PMC9494751
- DOI: 10.1021/acs.accounts.2c00470
Manganese Alkyl Carbonyl Complexes: From Iconic Stoichiometric Textbook Reactions to Catalytic Applications
Abstract
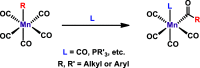
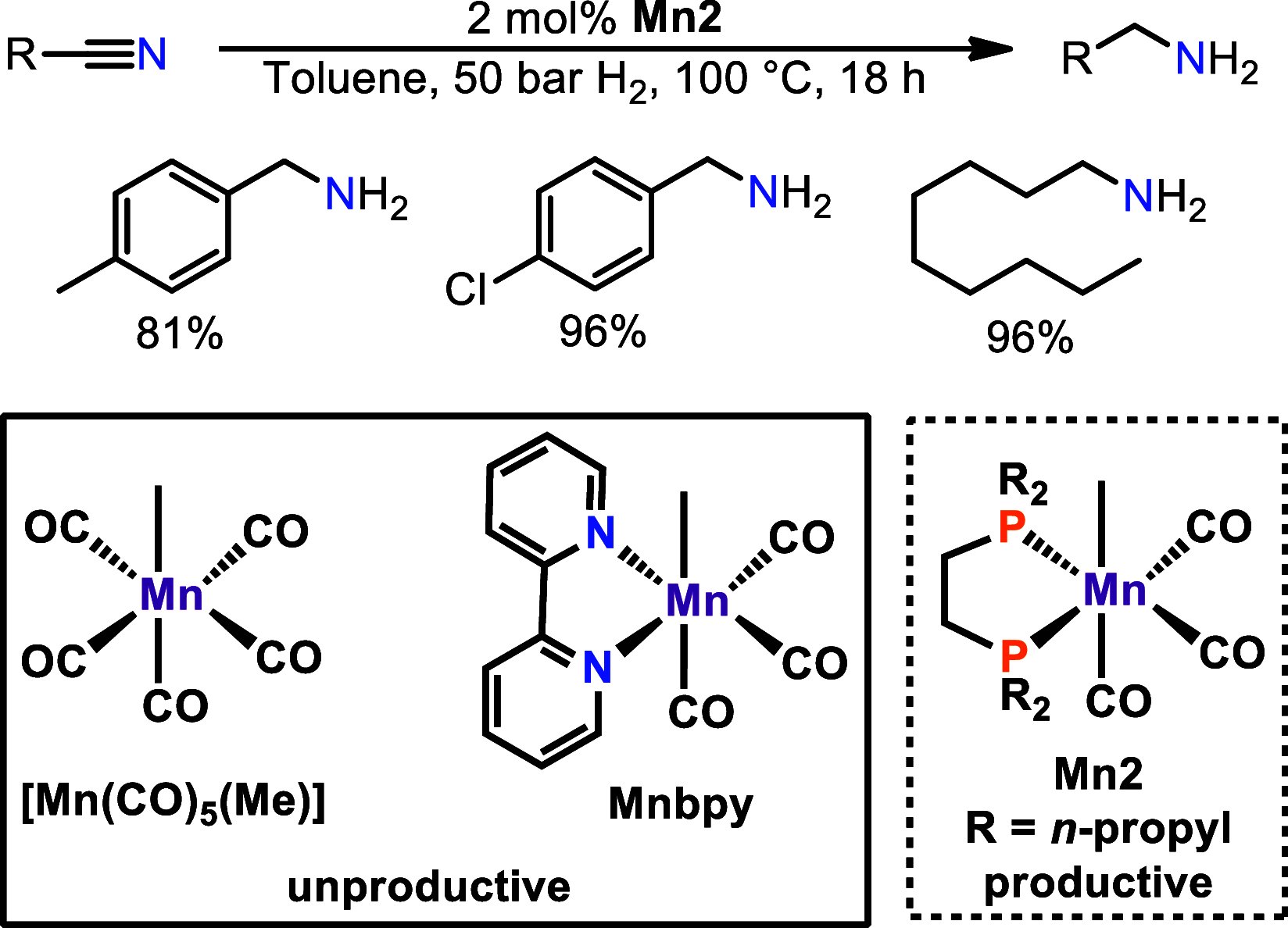
The activation of weakly polarized bonds represents a challenging, yet highly valuable process. In this context, precious metal catalysts have been used as reliable compounds for the activation of rather inert bonds for the last several decades. Nevertheless, base-metal complexes including cobalt, iron, or nickel are currently promising candidates for the substitution of noble metals in order to develop more sustainable processes. In the past few years, manganese(I)-based complexes were heavily employed as efficient catalysts for (de)hydrogenation reactions. However, the vast majority of these complexes operate via a metal-ligand bifunctionality as already well implemented for precious metals decades ago. Although high reactivity can be achieved in various reactions, this concept is often not applicable to certain transformations due to outer-sphere mechanisms. In this Account, we outline the potential of alkylated Mn(I)-carbonyl complexes for the activation of nonpolar and moderately polar E-H (E = H, B, C, Si) bonds and disclose our successful approach for the utilization of complexes in the field of homogeneous catalysis. This involves the rational design of manganese complexes for hydrogenation reactions involving ketones, nitriles, carbon dioxide, and alkynes. In addition to that, the reduction of alkenes by dihydrogen could be achieved by a series of well-defined manganese complexes which was not possible before. Furthermore, we elucidate the potential of our Mn-based catalysts in the field of hydrofunctionalization reactions for carbon-carbon multiple bonds. Our investigations unveiled novel insights into reaction pathways of dehydrogenative silylation of alkenes and trans-1,2-diboration of terminal alkynes, which was not yet reported for transition metals. Due to rational catalyst design, these transformations can be achieved under mild reaction conditions. Delightfully, all of the employed complexes are bench-stable compounds. We took advantage of the fact that Mn(I) alkyl complexes are known to undergo migratory insertion of the alkyl group into the CO ligand, yielding an unsaturated acyl intermediate. Hydrogen atom abstraction by the acyl ligand then paves the way to an active species for a variety of catalytic transformations which all proceed via an inner-sphere process. Although these textbook reactions have been well-known for decades, the application in catalytic transformations is still in its infancy. A brief historical overview of alkylated manganese(I)-carbonyl complexes is provided, covering the synthesis and especially iconic stoichiometric transformations, e.g., carbonylation, as intensively examined by Calderazzo, Moss, and others. An outline of potential future applications of defined alkyl manganese complexes will be given, which may inspire researchers for the development of novel (base-)metal catalysts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











References
-
- Weber S.; Stöger B.; Veiros L. F.; Kirchner K. Rethinking Basic Concepts - Hydrogenation of Alkenes Catalyzed by Bench-Stable Alkyl Mn(I) Complexes. ACS Catal. 2019, 9, 9715–9720. 10.1021/acscatal.9b03963. - DOI
-
- Campos J.; López-Serrano J.; Peloso R.; Carmona E. Methyl Complexes of the Transition Metals. Eur. Chem. J. 2016, 22, 6432–6457. 10.1002/chem.201504483. - DOI - PubMed
- Crabtree R. H.Alkyls and Hydrides. In The Organometallic Chemistry of the Transition Metals, 6th ed.; Wiley: Hoboken, 2014; pp 69–84.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

