Immunogenicity and safety of an inactivated whole-virus COVID-19 vaccine (VLA2001) compared with the adenoviral vector vaccine ChAdOx1-S in adults in the UK (COV-COMPARE): interim analysis of a randomised, controlled, phase 3, immunobridging trial
- PMID: 36075233
- PMCID: PMC9444237
- DOI: 10.1016/S1473-3099(22)00502-3
Immunogenicity and safety of an inactivated whole-virus COVID-19 vaccine (VLA2001) compared with the adenoviral vector vaccine ChAdOx1-S in adults in the UK (COV-COMPARE): interim analysis of a randomised, controlled, phase 3, immunobridging trial
Abstract
Background: The Valneva COVID-19 vaccine (VLA2001; Valneva Austria, Vienna, Austria) is an inactivated whole-virus, adjuvanted SARS-CoV-2 vaccine. We aimed to assess the safety and immunogenicity of primary vaccination with VLA2001 versus the ChAdOx1-S (Oxford-AstraZeneca) adenoviral-vectored vaccine.
Methods: In this immunobridging phase 3 trial (COV-COMPARE), participants aged 18 years and older who were medically stable (as determined by an investigator) were enrolled at 26 sites in the UK. In the double-blind, randomised, controlled arm of the trial, participants aged 30 years and older were randomly assigned (2:1) to receive two doses of VLA2001 (0·5 mL; with 33 antigen units [AU] per dose) or ChAdOx1-S (0·5 mL; with 2·5 × 108 infectious units per dose) on days 1 and 29. In another arm, participants aged 18-29 years received two doses of VLA2001 (same dose) open label on days 1 and 29. The primary immunogenicity outcome was the immune response of a two-dose schedule of VLA2001 on day 43, in adults aged 30 years and older, versus two doses of ChAdOx1-S via superiority of geometric mean titres (GMTs) of neutralising antibodies (GMT ratio of >1 at a two-sided significance level of 5%) and non-inferiority of the seroconversion rate (non-inferiority margin of -10% for the lower limit of the 95% CI for the difference between groups). The primary safety outcome was the frequency and severity of any adverse events in all participants up to day 43. Safety was assessed in all participants who received at least one dose of vaccine. GMTs were assessed in a subset of participants aged 30 years and older who were seronegative at baseline, had at least one evaluable antibody titre measurement after vaccination, and had no confirmed COVID-19 during the study (immunogenicity population); and seroconversion was assessed in the per-protocol population, which comprised the immunogenicity population but excluding any participants with major protocol violations. For each timepoint, only participants with available data were included in the analysis. This study is registered with ClinicalTrials.gov, NCT04864561, and is ongoing.
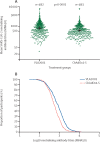
Findings: Between April 28 and June 3, 2021, 4181 individuals were screened and 4017 enrolled, of whom 2975 (74%) were aged 30 years or older and randomly assigned to receive VLA2001 (n=1978) or ChAdOx1-S (n=997), and 1042 (26%) were aged 18-29 years (all received open-label VLA2001). 4012 participants received at least one dose of vaccine (1040 in the open-label VLA2001 group, 1977 in the randomised VLA2001 group, and 995 in the ChAdOx1-S group). The immunogenicity population comprised 492 participants in the randomised VLA2001 group and 498 in the ChAdOx1-S group; three participants in the VLA2001 group were excluded from the per-protocol population. VLA2001 induced higher neutralising GMTs than did ChAdOx1-S (803·5 [95% CI 748·5-862·6] vs 576·6 [543·6-611·7]; GMT ratio 1·39 [95% CI 1·25-1·56]; p<0·0001), and non-inferior seroconversion rates (444 [97·4%] of 456 participants vs 444 [98·9%] of 449; difference -1·5% [95% CI -3·3 to 0·2]. Any adverse event was reported in 963 (92·6%) participants in the open-label VLA2001 group, 1755 (88·8%) in the randomised VLA2001 group, and 976 (98·1%) in the ChAdOx1-S group. Most adverse events reported were mild or moderate in severity.
Interpretation: VLA2001 has a favourable tolerability profile and met superiority criteria for neutralising antibodies and non-inferiority criterion for seroconversion rates compared with ChAdOx1-S. The data presented here formed the basis of successful marketing approval for use of VLA2001 in primary vaccination in the EU, the UK, Bahrain, and United Arab Emirates.
Funding: UK Department of Health and Social Care and Valneva Austria.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AF is a member of the Joint Committee on Vaccination and Immunisation, chair of the WHO European Technical Advisory Group of Experts on Immunisation, member of the WHO Working Group on COVID19 vaccines and is an investigator or provides consulting advice on clinical trials and studies of COVID-19 vaccines produced by AstraZeneca, Janssen, Valneva, Pfizer, Moderna, and Sanofi, and of other vaccines from these and other manufacturers, including GlaxoSmithKline, VPI Pharmaceuticals, Takeda, and Bionet Asia; he receives no personal remuneration or benefits for any of this work. ICR, KD, SD, SE-L, RH, JCJ, MK, BQ, CT, and PW are employees of Valneva Austria GmbH. RL has received grants or worked on clinical trials funded by Valneva, Moderna, Janssen, and AstraZeneca and has received support for meeting attendance, lectures, or writing from AstraZeneca.
Figures
Comment in
-
Evaluating novel COVID-19 vaccines in the current chapter of the pandemic.Lancet Infect Dis. 2022 Dec;22(12):1652-1654. doi: 10.1016/S1473-3099(22)00517-5. Epub 2022 Sep 5. Lancet Infect Dis. 2022. PMID: 36075232 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

