Dipeptidyl peptidase-1 inhibition in patients hospitalised with COVID-19: a multicentre, double-blind, randomised, parallel-group, placebo-controlled trial
- PMID: 36075243
- PMCID: PMC9442496
- DOI: 10.1016/S2213-2600(22)00261-2
Dipeptidyl peptidase-1 inhibition in patients hospitalised with COVID-19: a multicentre, double-blind, randomised, parallel-group, placebo-controlled trial
Abstract
Background: Neutrophil serine proteases are involved in the pathogenesis of COVID-19 and increased serine protease activity has been reported in severe and fatal infection. We investigated whether brensocatib, an inhibitor of dipeptidyl peptidase-1 (DPP-1; an enzyme responsible for the activation of neutrophil serine proteases), would improve outcomes in patients hospitalised with COVID-19.
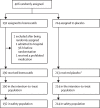
Methods: In a multicentre, double-blind, randomised, parallel-group, placebo-controlled trial, across 14 hospitals in the UK, patients aged 16 years and older who were hospitalised with COVID-19 and had at least one risk factor for severe disease were randomly assigned 1:1, within 96 h of hospital admission, to once-daily brensocatib 25 mg or placebo orally for 28 days. Patients were randomly assigned via a central web-based randomisation system (TruST). Randomisation was stratified by site and age (65 years or ≥65 years), and within each stratum, blocks were of random sizes of two, four, or six patients. Participants in both groups continued to receive other therapies required to manage their condition. Participants, study staff, and investigators were masked to the study assignment. The primary outcome was the 7-point WHO ordinal scale for clinical status at day 29 after random assignment. The intention-to-treat population included all patients who were randomly assigned and met the enrolment criteria. The safety population included all participants who received at least one dose of study medication. This study was registered with the ISRCTN registry, ISRCTN30564012.
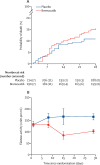
Findings: Between June 5, 2020, and Jan 25, 2021, 406 patients were randomly assigned to brensocatib or placebo; 192 (47·3%) to the brensocatib group and 214 (52·7%) to the placebo group. Two participants were excluded after being randomly assigned in the brensocatib group (214 patients included in the placebo group and 190 included in the brensocatib group in the intention-to-treat population). Primary outcome data was unavailable for six patients (three in the brensocatib group and three in the placebo group). Patients in the brensocatib group had worse clinical status at day 29 after being randomly assigned than those in the placebo group (adjusted odds ratio 0·72 [95% CI 0·57-0·92]). Prespecified subgroup analyses of the primary outcome supported the primary results. 185 participants reported at least one adverse event; 99 (46%) in the placebo group and 86 (45%) in the brensocatib group. The most common adverse events were gastrointestinal disorders and infections. One death in the placebo group was judged as possibly related to study drug.
Interpretation: Brensocatib treatment did not improve clinical status at day 29 in patients hospitalised with COVID-19.
Funding: Sponsored by the University of Dundee and supported through an Investigator Initiated Research award from Insmed, Bridgewater, NJ; STOP-COVID19 trial.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests JDC reports grants and personal fees from AstraZeneca, Boehringer-Ingelheim, Chiesi, GSK, Gilead Sciences, Grifols, Insmed, Janssen, Novartis, and Zambon. CB reports grants from the UK National Institute for Health and Care Research Biomedical Research Centre during the conduct of the study; grants and personal fees from GSK, AstraZeneca, Chiesi, Boehringer-Ingelheim, Genentech, Roche, Sanofi, Regeneron, Merck, TEVA, Mologic, 4DPharma, and Novartis. AART reports grants and personal fees from British Heart Foundation and Actelion Pharmaceuticals. JU reports personal fees from Gilead Sciences and ViiV Healthcare and from Celltrion; and is supported by the UK Medical Research Council (MR/T023791/1). DPSD reports grants and personal fees from GSK, Vir Biotechnology, AstraZeneca, and Boehringer-Ingelheim. ASm has received non-financial support for clinical trial work from AstraZeneca, GSK, Chiesi, and Oncimmune; and has done consultancy work with AstraZeneca and GSK. MP reports non-financial support for clinical trial work from AstraZeneca, GSK, Chiesi, and Oncimmune and consultancy work with AstraZeneca and GSK. All other authors report no competing interests.
Figures
Comment in
-
Is neutrophilic inflammation treatable in COVID-19?Lancet Respir Med. 2022 Dec;10(12):1100-1101. doi: 10.1016/S2213-2600(22)00293-4. Epub 2022 Sep 5. Lancet Respir Med. 2022. PMID: 36075244 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

