Tracing the lactate shuttle to the mitochondrial reticulum
- PMID: 36075947
- PMCID: PMC9534995
- DOI: 10.1038/s12276-022-00802-3
Tracing the lactate shuttle to the mitochondrial reticulum
Abstract
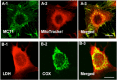
Isotope tracer infusion studies employing lactate, glucose, glycerol, and fatty acid isotope tracers were central to the deduction and demonstration of the Lactate Shuttle at the whole-body level. In concert with the ability to perform tissue metabolite concentration measurements, as well as determinations of unidirectional and net metabolite exchanges by means of arterial-venous difference (a-v) and blood flow measurements across tissue beds including skeletal muscle, the heart and the brain, lactate shuttling within organs and tissues was made evident. From an extensive body of work on men and women, resting or exercising, before or after endurance training, at sea level or high altitude, we now know that Organ-Organ, Cell-Cell, and Intracellular Lactate Shuttles operate continuously. By means of lactate shuttling, fuel-energy substrates can be exchanged between producer (driver) cells, such as those in skeletal muscle, and consumer (recipient) cells, such as those in the brain, heart, muscle, liver and kidneys. Within tissues, lactate can be exchanged between white and red fibers within a muscle bed and between astrocytes and neurons in the brain. Within cells, lactate can be exchanged between the cytosol and mitochondria and between the cytosol and peroxisomes. Lactate shuttling between driver and recipient cells depends on concentration gradients created by the mitochondrial respiratory apparatus in recipient cells for oxidative disposal of lactate.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures