A Retrospective Real-World Study of the Effectiveness and Tolerability of Tildrakizumab in UK Adults with Moderate-to-Severe Chronic Plaque Psoriasis
- PMID: 36076145
- PMCID: PMC9458480
- DOI: 10.1007/s13555-022-00800-3
A Retrospective Real-World Study of the Effectiveness and Tolerability of Tildrakizumab in UK Adults with Moderate-to-Severe Chronic Plaque Psoriasis
Abstract
Introduction: As with most medicines historically, clinicians prescribing tildrakizumab have relied on information derived from registration studies undertaken in a prospective controlled clinical trial setting. More recently, clinicians, policymakers, and commissioners increasingly rely on real-world data to inform both policy and practice.
Methods: A retrospective real-world data study was undertaken at four specialist dermatology departments in the United Kingdom. All adult patients treated with tildrakizumab for moderate-to-severe plaque psoriasis were included, with data being collected for 122 patients.
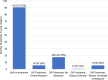
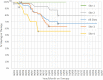
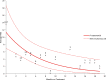
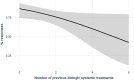
Results: Psoriatic patients on tildrakizumab tended to be overweight (median body mass index of 32 (range 19-59) (n = 61); 26/68 (38%) < 90 kg, 32/68 (47%) between 90 and 120 kg, and 10/68 (15%) > 120 kg). The study population had high levels of comorbidities (83/116, 72%), multiple special sites (39/117, 33%), and histories of biological treatments (81/100, 81%). Most patients (61/80, 76%) initiated on tildrakizumab were switched from another biological treatment. Tildrakizumab was effective, with 91/122 (75%) patients remaining on treatment for the duration of the study-a median of 12 months per patient (range 1-29 months)-and achieving a change in median Psoriasis Area and Severity Index (PASI) from 12 to 0.35 and in Dermatology Life Quality Index (DLQI) from 20 to 0. The response rate was 57/66 (86%) when tildrakizumab was used as the first- or second-line biologic compared to 19/31 (61%) when used as the third- to seventh-line. Thirty-three (78.6%) patients over 90 kg of weight received the 200-mg dose of tildrakizumab. All but one (n = 8) patient with body weight over 120 kg maintained response over time. There was one treatment discontinuation; a patient who had a local sensitivity reaction.
Conclusions: In UK clinical practice, tildrakizumab was well tolerated and effective at doses of 100 mg or 200 mg in a range of patient phenotypes.
Keywords: Plaque psoriasis; Real world; Tildrakizumab.
© 2022. The Author(s).
Figures
References
-
- WHO. World psoriasis day-document EB133.R2, agenda item 6.2. 2013. https://apps.who.int/gb/ebwha/pdf_files/EB133/B133_R2-en.pdf. Accessed 14 Apr 2022.
LinkOut - more resources
Full Text Sources
Research Materials

