Evolutionary changes in gene expression profiles associated with the coevolution of male and female genital parts among closely related ground beetle species
- PMID: 36076166
- PMCID: PMC9454128
- DOI: 10.1186/s12864-022-08865-2
Evolutionary changes in gene expression profiles associated with the coevolution of male and female genital parts among closely related ground beetle species
Abstract
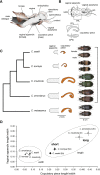
Background: The coevolutionary dynamics of corresponding male and female sexual traits, including genitalia, may be driven by complex genetic mechanisms. Carabus (Ohomopterus) ground beetles show correlated evolution in the size of their functionally corresponding male and female genital parts. To reveal the genetic mechanisms involved in the evolution of size, we investigated interspecific differences in gene expression profiles in four closely related species (two species each with long and short genital parts) using transcriptome data from genital tissues in the early and late pupal stages.
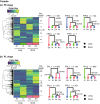
Results: We detected 1536 and 1306 differentially expressed genes (DEGs) among the species in males and 546 and 1959 DEGs in females in the two pupal stages, respectively. The DEGs were clustered by species-specific expression profiles for each stage and sex to identify candidate gene clusters for genital size based on the expression patterns among the species and gene ontology. We identified one and two gene clusters in females and males, respectively, all from the late pupal stage; one cluster of each sex showed similar expression profiles in species with similar genital size, which implies a common gene expression change associated with similar genital size in each sex. However, the remaining male cluster showed different expression profiles between species with long genital parts, which implies species-specific gene expression changes. These clusters did not show sex-concordant expression profiles for genital size differences.
Conclusion: Our study demonstrates that sex-independent and partly species-specific gene expression underlies the correlated evolution of male and female genital size. These results may reflect the complex evolutionary history of male and female genitalia.
Keywords: Character evolution; Differentially expressed genes; Genital formation; Interspecific differences; Sexual traits; Transcriptome.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Fisher RA. The genetical theory of natural selection. Oxford: Clarendon Press; 1930.
-
- Servedio MR, Boughman JW. The role of sexual selection in local adaptation and speciation. Annu Rev Ecol Evol Syst. 2017;48:85–109. doi: 10.1146/annurev-ecolsys-110316-022905. - DOI

