Production of surfactant-stable keratinase from Bacillus cereus YQ15 and its application as detergent additive
- PMID: 36076195
- PMCID: PMC9454225
- DOI: 10.1186/s12896-022-00757-3
Production of surfactant-stable keratinase from Bacillus cereus YQ15 and its application as detergent additive
Abstract
Background: With the growing concern for the environment, there are trends that bio-utilization of keratinous waste by keratinases could ease the heavy burden of keratinous waste from the poultry processing and leather industry. Especially surfactant-stable keratinases are beneficial for the detergent industry. Therefore, the production of keratinase by Bacillus cereus YQ15 was improved; the characterization and use of keratinase in detergent were also studied.
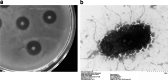
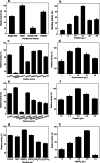
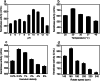
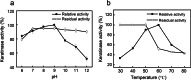
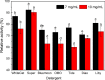
Results: A novel alkaline keratinase-producing bacterium YQ15 was isolated from feather keratin-rich soil and was identified as Bacillus cereus. Based on the improvement of medium components and culture conditions, the maximum keratinase activity (925 U/mL) was obtained after 36 h of cultivation under conditions of 35 °C and 160 rpm. Moreover, it was observed that the optimal reacting temperature and pH of the keratinase are 60 °C and 10.0, respectively; the activity was severely inhibited by PMSF and EDTA. On the contrary, the keratinase showed remarkable stability in the existence of the various surfactants, including SDS, Tween 20, Tween 60, Tween 80, and Triton X-100. Especially, 5% of Tween 20 and Tween 60 increased the activity by 100% and 60%, respectively. Furtherly, the keratinase revealed high efficiency in removing blood stains.
Conclusion: The excellent compatibility with commercial detergents and the high washing efficiency of removing blood stains suggested its suitability for potential application as a bio-detergent additive.
Keywords: Bacillus cereus; Detergent compatibility; Keratinase; Production.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

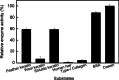

Similar articles
-
Sustainable production, biochemical and molecular characterization of thermo-and-solvent stable alkaline serine keratinase from novel Bacillus pumilus AR57 for promising poultry solid waste management.Int J Biol Macromol. 2020 Nov 15;163:135-146. doi: 10.1016/j.ijbiomac.2020.06.219. Epub 2020 Jun 29. Int J Biol Macromol. 2020. PMID: 32615225
-
Production and characterization of a novel thermo- and detergent stable keratinase from Bacillus sp. NKSP-7 with perceptible applications in leather processing and laundry industries.Int J Biol Macromol. 2020 Dec 1;164:371-383. doi: 10.1016/j.ijbiomac.2020.07.146. Epub 2020 Jul 17. Int J Biol Macromol. 2020. PMID: 32682971
-
Bacillus sp. CSK2 produced thermostable alkaline keratinase using agro-wastes: keratinolytic enzyme characterization.BMC Biotechnol. 2020 Dec 14;20(1):65. doi: 10.1186/s12896-020-00659-2. BMC Biotechnol. 2020. PMID: 33317483 Free PMC article.
-
Research progress on the degradation mechanism and modification of keratinase.Appl Microbiol Biotechnol. 2023 Feb;107(4):1003-1017. doi: 10.1007/s00253-023-12360-3. Epub 2023 Jan 12. Appl Microbiol Biotechnol. 2023. PMID: 36633625 Review.
-
Comprehensive insights into microbial keratinases and their implication in various biotechnological and industrial sectors: A review.Int J Biol Macromol. 2020 Jul 1;154:567-583. doi: 10.1016/j.ijbiomac.2020.03.116. Epub 2020 Mar 16. Int J Biol Macromol. 2020. PMID: 32194110 Review.
Cited by
-
Thermoactive metallo-keratinase from Bacillus sp. NFH5: Characterization, structural elucidation, and potential application as detergent additive.Heliyon. 2023 Feb 13;9(2):e13635. doi: 10.1016/j.heliyon.2023.e13635. eCollection 2023 Feb. Heliyon. 2023. PMID: 36852054 Free PMC article.
-
Strawberry Protease as a Laundry Detergent Additive Candidate: Immobilization, Compatibility Study with Detergent Ingredients, and Washing Performance Test.Glob Chall. 2023 Nov 24;8(1):2300102. doi: 10.1002/gch2.202300102. eCollection 2024 Jan. Glob Chall. 2023. PMID: 38223888 Free PMC article.
-
Synthesis and characterization of keratinase laden green synthesized silver nanoparticles for valorization of feather keratin.Sci Rep. 2023 Jul 18;13(1):11608. doi: 10.1038/s41598-023-38721-6. Sci Rep. 2023. PMID: 37463953 Free PMC article.
-
Biochemical and molecular characterization of novel keratinolytic protease from Bacillus licheniformis (KRLr1).Front Microbiol. 2023 May 10;14:1132760. doi: 10.3389/fmicb.2023.1132760. eCollection 2023. Front Microbiol. 2023. PMID: 37234543 Free PMC article.
-
New Bacillus paralicheniformis strain with high proteolytic and keratinolytic activity.Sci Rep. 2024 Sep 30;14(1):22621. doi: 10.1038/s41598-024-73468-8. Sci Rep. 2024. PMID: 39349615 Free PMC article.
References
-
- Paul T, Jana A, Mandal AK, Mandal A, Mohpatra PKD, Mondal KC. Bacterial keratinolytic protease, imminent starter for NextGen leather and detergent industries. Sustain Chem Pharm. 2016;3:8–22. doi: 10.1016/j.scp.2016.01.001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

