Intravenous versus oral iron for iron deficiency anaemia in pregnant Nigerian women (IVON): study protocol for a randomised hybrid effectiveness-implementation trial
- PMID: 36076211
- PMCID: PMC9454388
- DOI: 10.1186/s13063-022-06690-2
Intravenous versus oral iron for iron deficiency anaemia in pregnant Nigerian women (IVON): study protocol for a randomised hybrid effectiveness-implementation trial
Abstract
Background: Anaemia in pregnancy is highly prevalent in African countries. High-dose oral iron is the current recommended treatment for pregnancy-related iron deficiency anaemia (IDA) in Nigeria and other African countries. This oral regimen is often poorly tolerated and has several side effects. Parenteral iron preparations are now available for the treatment of IDA in pregnancy but not widely used in Africa. The IVON trial is investigating the comparative effectiveness and safety of intravenous ferric carboxymaltose versus oral ferrous sulphate standard-of-care for pregnancy-related IDA in Nigeria. We will also measure the implementation outcomes of acceptability, feasibility, fidelity, and cost-effectiveness for intravenous ferric carboxymaltose.
Methods: This is an open-label randomised controlled trial with a hybrid type 1 effectiveness-implementation design, conducted at 10 health facilities in Kano (Northern) and Lagos (Southern) states in Nigeria. A total of 1056 pregnant women at 20-32 weeks' gestational age with moderate or severe anaemia (Hb < 10g/dl) will be randomised 1:1 into two groups. The interventional treatment is one 1000-mg dose of intravenous ferric carboxymaltose at enrolment; the control treatment is thrice daily oral ferrous sulphate (195 mg elemental iron daily), from enrolment till 6 weeks postpartum. Primary outcome measures are (1) the prevalence of maternal anaemia at 36 weeks and (2) infant preterm birth (<37 weeks' gestation) and will be analysed by intention-to-treat. Maternal full blood count and iron panel will be assayed at 4 weeks post-enrolment, 36 weeks' gestation, delivery, and 6 weeks postpartum. Implementation outcomes of acceptability, feasibility, fidelity, and cost will be assessed with structured questionnaires, key informant interviews, and focus group discussions.
Discussion: The IVON trial could provide both effectiveness and implementation evidence to guide policy for integration and uptake of intravenous iron for treating anaemia in pregnancy in Nigeria and similar resource-limited, high-burden settings. If found effective, further studies exploring different intravenous iron doses are planned.
Trial registration: ISRCTN registry ISRCTN63484804 . Registered on 10 December 2020 Clinicaltrials.gov NCT04976179 . Registered on 26 July 2021 The current protocol version is version 2.1 (080/080/2021).
Keywords: Anaemia in pregnancy; Anaemia, iron deficiency; Cost-effectiveness; Depression; Ferric carboxymaltose; Ferrous sulphate; Implementation science; Pregnancy; Protocol.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

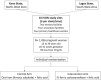

 denotes variables that will be assessed at each visit from the selected time point (X) to the end point depicted by the double-headed arrow. Acceptability of intervention will be assessed pre-trial, and findings used in protocol modification
denotes variables that will be assessed at each visit from the selected time point (X) to the end point depicted by the double-headed arrow. Acceptability of intervention will be assessed pre-trial, and findings used in protocol modificationReferences
-
- WHO . Anaemia. Nutrition landscape information system (NLiS) 2022.
-
- Noronha JA, Khasawneh EA, Seshan V, Ramasubramaniam S, Raman S. Anemia in pregnancy-consequences and challenges: a review of literature. J SAFOG. 2012;4:64–70. doi: 10.5005/jp-journals-10006-1177. - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

