YAP and TAZ play a crucial role in human erythrocyte maturation and enucleation
- PMID: 36076260
- PMCID: PMC9461202
- DOI: 10.1186/s13287-022-03166-7
YAP and TAZ play a crucial role in human erythrocyte maturation and enucleation
Abstract
Background: Yes-associated protein (YAP) and WW domain-containing transcription regulator protein 1 (WWTR1, also known as TAZ) are two key transcription co-activators of the Hippo pathway. Both were originally characterized as organ size and cell proliferation regulators. Later studies demonstrated that the Hippo pathway may play a role in Drosophila and mammal hematopoiesis. However, the role of the Hippo pathway in human erythropoiesis has not yet been fully elucidated.
Methods: The role of YAP and TAZ was studied in human erythropoiesis and hematopoietic stem cell (HSC) lineage determination by using mobilized peripheral blood (PB) and cord blood (CB)-derived HSC as a model. HSCs were isolated and cultured in an erythroid differentiation medium for erythroid differentiation and culture in methylcellulose assay for HSC lineage determination study.
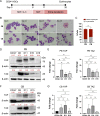
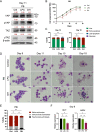
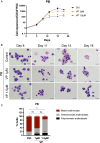
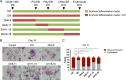
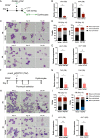
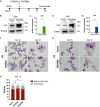
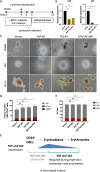
Results: YAP and TAZ were barely detectable in human HSCs, but became highly expressed in pro-erythroblasts and erythroblasts. Depletion or knockdown of YAP and/or TAZ did not affect the ability of HSC lineage specification to erythroid lineage in either methylcellulose assay or liquid culture. However, depletion of YAP and TAZ did impair erythroblast terminal differentiation to erythrocytes and their enucleation. Moreover, ectopic expression of YAP and TAZ in pro-erythroblasts did not exert an apparent effect on erythroid differentiation, expansion, or morphology.
Conclusions: This study demonstrated that YAP/TAZ plays important role in erythroid maturation and enucleation but is dispensable for lineage determination of human HSCs.
Keywords: Erythroid differentiation; Hematopoietic stem cells; Hippo pathway; TAZ; YAP.
© 2022. The Author(s).
Conflict of interest statement
All authors declare no personal or professional conflicts of interest relating to any aspect of this study.
Figures
Similar articles
-
Role of the Hippo-YAP/TAZ Pathway in Epithelioid Hemangioendothelioma and its Potential as a Therapeutic Target.Anticancer Res. 2024 Oct;44(10):4147-4153. doi: 10.21873/anticanres.17245. Anticancer Res. 2024. PMID: 39348982 Review.
-
Nuclear phosphoinositide signaling promotes YAP/TAZ-TEAD transcriptional activity in breast cancer.EMBO J. 2024 May;43(9):1740-1769. doi: 10.1038/s44318-024-00085-6. Epub 2024 Apr 2. EMBO J. 2024. PMID: 38565949 Free PMC article.
-
Hippo pathway-mediated YAP1/TAZ inhibition is essential for proper pancreatic endocrine specification and differentiation.Elife. 2024 Jul 25;13:e84532. doi: 10.7554/eLife.84532. Elife. 2024. PMID: 39051998 Free PMC article.
-
Hippo pathway effectors YAP, TAZ and TEAD are associated with EMT master regulators ZEB, Snail and with aggressive phenotype in phyllodes breast tumors.Pathol Res Pract. 2024 Oct;262:155551. doi: 10.1016/j.prp.2024.155551. Epub 2024 Aug 15. Pathol Res Pract. 2024. PMID: 39153238
-
Non-hippo kinases: indispensable roles in YAP/TAZ signaling and implications in cancer therapy.Mol Biol Rep. 2023 May;50(5):4565-4578. doi: 10.1007/s11033-023-08329-0. Epub 2023 Mar 6. Mol Biol Rep. 2023. PMID: 36877351 Review.
Cited by
-
Role of YAP in hematopoietic differentiation and erythroid lineage specification of human-induced pluripotent stem cells.Stem Cell Res Ther. 2023 Sep 29;14(1):279. doi: 10.1186/s13287-023-03508-z. Stem Cell Res Ther. 2023. PMID: 37775798 Free PMC article.
-
Repurposing of an inotropic drug dobutamine to enhance the production of human hematopoietic stem cells from human induced pluripotent stem cells.Stem Cell Res Ther. 2025 Jun 9;16(1):298. doi: 10.1186/s13287-025-04427-x. Stem Cell Res Ther. 2025. PMID: 40490826 Free PMC article.
-
Identification and validation of ferroptosis related markers in erythrocyte differentiation of umbilical cord blood-derived CD34+ cell by bioinformatic analysis.Front Genet. 2024 Jul 30;15:1365232. doi: 10.3389/fgene.2024.1365232. eCollection 2024. Front Genet. 2024. PMID: 39139819 Free PMC article.
-
Role of YAP as a Mechanosensing Molecule in Stem Cells and Stem Cell-Derived Hematopoietic Cells.Int J Mol Sci. 2022 Nov 23;23(23):14634. doi: 10.3390/ijms232314634. Int J Mol Sci. 2022. PMID: 36498961 Free PMC article. Review.
-
Emerging role of Hippo pathway in the regulation of hematopoiesis.BMB Rep. 2023 Aug;56(8):417-425. doi: 10.5483/BMBRep.2023-0094. BMB Rep. 2023. PMID: 37574808 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

