The Core Rehabilitation Outcome Set for Single-Sided Deafness (CROSSSD) study: International consensus on outcome measures for trials of interventions for adults with single-sided deafness
- PMID: 36076299
- PMCID: PMC9454406
- DOI: 10.1186/s13063-022-06702-1
The Core Rehabilitation Outcome Set for Single-Sided Deafness (CROSSSD) study: International consensus on outcome measures for trials of interventions for adults with single-sided deafness
Abstract
Background: Single-sided deafness (SSD) has functional, psychological, and social consequences. Interventions for adults with SSD include hearing aids and auditory implants. Benefits and harms (outcome domains) of these interventions are until now reported inconsistently in clinical trials. Inconsistency in reporting outcome measures prevents meaningful comparisons or syntheses of trial results. The Core Rehabilitation Outcome Set for Single-Sided Deafness (CROSSSD) international initiative used structured communication techniques to achieve consensus among healthcare users and professionals working in the field of SSD. The novel contribution is a set of core outcome domains that experts agree are critically important to assess in all clinical trials of SSD interventions.
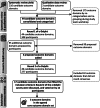
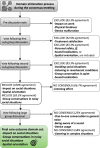
Methods: A long list of candidate outcome domains compiled from a systematic review and published qualitative data, informed the content of a two-round online Delphi survey. Overall, 308 participants from 29 countries were enrolled. Of those, 233 participants completed both rounds of the survey and scored each outcome domain on a 9-point scale. The set of core outcome domains was finalised via a web-based consensus meeting with 12 participants. Votes involved all stakeholder groups, with an approximate 2:1 ratio of professionals to healthcare users participating in the Delphi survey, and a 1:1 ratio participating in the consensus meeting.
Results: The first round of the survey listed 44 potential outcome domains, organised thematically. A further five outcome domains were included in Round 2 based on participant feedback. The structured voting at round 2 identified 17 candidate outcome domains which were voted on at the consensus meeting. Consensus was reached for a core outcome domain set including three outcome domains: spatial orientation, group conversations in noisy social situations, and impact on social situations. Seventy-seven percent of the remaining Delphi participants agreed with this core outcome domain set.
Conclusions: Adoption of the internationally agreed core outcome domain set would promote consistent assessment and reporting of outcomes that are meaningful and important to all relevant stakeholders. This consistency will in turn enable comparison of outcomes reported across clinical trials comparing SSD interventions in adults and reduce research waste. Further research will determine how those outcome domains should best be measured.
Keywords: Clinical trial design; Consensus methods; Core outcome set; Hearing interventions; Outcome domains; Single-sided deafness.
© 2022. The Author(s).
Conflict of interest statement
PTK declares receiving research grants and support in kind awarded to his institution for research projects in the field of SSD from manufacturers of hearing devices. PHVH declares research grants to his institution (UZA) from MED-EL (Innsbruck, Austria) and Cochlear® (Basel, Switzerland). JBF declares research grants to her institution from Cochlear® Americas (Lone Tree, Colorado) and Advanced Bionics (Valencia, California). All other authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

