Recent advances in point of care testing for COVID-19 detection
- PMID: 36076617
- PMCID: PMC9371983
- DOI: 10.1016/j.biopha.2022.113538
Recent advances in point of care testing for COVID-19 detection
Abstract
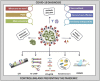
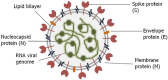
The World Health Organizations declaration of the COVID-19 pandemic was a milestone for the scientific community. The high transmission rate and the huge number of deaths, along with the lack of knowledge about the virus and the evolution of the disease, stimulated a relentless search for diagnostic tests, treatments, and vaccines. The main challenges were the differential diagnosis of COVID-19 and the development of specific, rapid, and sensitive tests that could reach all people. RT-PCR remains the gold standard for diagnosing COVID-19. However, new methods, such as other molecular techniques and immunoassays emerged. Also, the need for accessible tests with quick results boosted the development of point of care tests (POCT) that are fast, and automated, with high precision and accuracy. This assay reduces the dependence on laboratory conditions and mass testing of the population, dispersing the pressure regarding screening and detection. This review summarizes the advances in the diagnostic field since the pandemic started, emphasizing various laboratory techniques for detecting COVID-19. We reviewed the main existing diagnostic methods, as well as POCT under development, starting with RT-PCR detection, but also exploring other nucleic acid techniques, such as digital PCR, loop-mediated isothermal amplification-based assay (RT-LAMP), clustered regularly interspaced short palindromic repeats (CRISPR), and next-generation sequencing (NGS), and immunoassay tests, and nanoparticle-based biosensors, developed as portable instruments for the rapid standard diagnosis of COVID-19.
Keywords: COVID-19; CRISPR; Diagnosis; Immunoassay; Nanobiosensors; Point-of-care testing; RT-LAMP; RT-PCR; SARS-CoV-2.
Copyright © 2022 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Conflict of interest statement The authors declare that they have no conflict of interest.
Figures



Similar articles
-
A Recent Update on Advanced Molecular Diagnostic Techniques for COVID-19 Pandemic: An Overview.Front Immunol. 2021 Dec 14;12:732756. doi: 10.3389/fimmu.2021.732756. eCollection 2021. Front Immunol. 2021. PMID: 34970254 Free PMC article. Review.
-
CLEVER assay: A visual and rapid RNA extraction-free detection of SARS-CoV-2 based on CRISPR-Cas integrated RT-LAMP technology.J Appl Microbiol. 2022 Aug;133(2):410-421. doi: 10.1111/jam.15571. Epub 2022 Apr 18. J Appl Microbiol. 2022. PMID: 35396760 Free PMC article.
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
iSCAN: An RT-LAMP-coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-2.Virus Res. 2020 Oct 15;288:198129. doi: 10.1016/j.virusres.2020.198129. Epub 2020 Aug 18. Virus Res. 2020. PMID: 32822689 Free PMC article.
-
Recent advances in methods for the diagnosis of Corona Virus Disease 2019.J Clin Lab Anal. 2022 Jan;36(1):e24178. doi: 10.1002/jcla.24178. Epub 2021 Dec 17. J Clin Lab Anal. 2022. PMID: 34921443 Free PMC article. Review.
Cited by
-
Evaluation and comparison of one-step real-time PCR and one-step RT-LAMP methods for detection of SARS-CoV-2.BMC Infect Dis. 2024 Jul 9;24(1):679. doi: 10.1186/s12879-024-09574-9. BMC Infect Dis. 2024. PMID: 38982392 Free PMC article.
-
Recent Advances in Quantum Dot-Based Lateral Flow Immunoassays for the Rapid, Point-of-Care Diagnosis of COVID-19.Biosensors (Basel). 2023 Aug 3;13(8):786. doi: 10.3390/bios13080786. Biosensors (Basel). 2023. PMID: 37622872 Free PMC article. Review.
-
Applications of Mass Spectrometry in the Characterization, Screening, Diagnosis, and Prognosis of COVID-19.Adv Exp Med Biol. 2024;1443:33-61. doi: 10.1007/978-3-031-50624-6_3. Adv Exp Med Biol. 2024. PMID: 38409415
-
Bacterial Proteases as Potentially Exploitable Modulators of SARS-CoV-2 Infection: Logic from the Literature, Informatics, and Inspiration from the Dog.BioTech (Basel). 2023 Oct 30;12(4):61. doi: 10.3390/biotech12040061. BioTech (Basel). 2023. PMID: 37987478 Free PMC article.
-
Visualization of Critical Limits and Critical Values Facilitates Interpretation.Diagnostics (Basel). 2025 Mar 2;15(5):604. doi: 10.3390/diagnostics15050604. Diagnostics (Basel). 2025. PMID: 40075851 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

