The Acute Antiallodynic Effect of Tolperisone in Rat Neuropathic Pain and Evaluation of Its Mechanism of Action
- PMID: 36076962
- PMCID: PMC9455595
- DOI: 10.3390/ijms23179564
The Acute Antiallodynic Effect of Tolperisone in Rat Neuropathic Pain and Evaluation of Its Mechanism of Action
Abstract
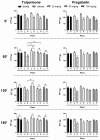
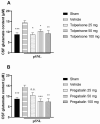
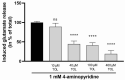
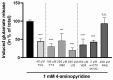
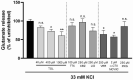
Current treatment approaches to manage neuropathic pain have a slow onset and their use is largely hampered by side-effects, thus there is a significant need for finding new medications. Tolperisone, a centrally acting muscle relaxant with a favorable side effect profile, has been reported to affect ion channels, which are targets for current first-line medications in neuropathic pain. Our aim was to explore its antinociceptive potency in rats developing neuropathic pain evoked by partial sciatic nerve ligation and the mechanisms involved. Acute oral tolperisone restores both the decreased paw pressure threshold and the elevated glutamate level in cerebrospinal fluid in neuropathic rats. These effects were comparable to those of pregabalin, a first-line medication in neuropathy. Tolperisone also inhibits release of glutamate from rat brain synaptosomes primarily by blockade of voltage-dependent sodium channels, although inhibition of calcium channels may also be involved at higher concentrations. However, pregabalin fails to affect glutamate release under our present conditions, indicating a different mechanism of action. These results lay the foundation of the avenue for repurposing tolperisone as an analgesic drug to relieve neuropathic pain.
Keywords: CSF glutamate content; neuronal glutamate release; neuropathic pain; pregabalin; synaptosome; tolperisone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

