Monoclonal Antibody Engineering and Design to Modulate FcRn Activities: A Comprehensive Review
- PMID: 36077002
- PMCID: PMC9455995
- DOI: 10.3390/ijms23179604
Monoclonal Antibody Engineering and Design to Modulate FcRn Activities: A Comprehensive Review
Abstract
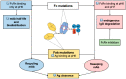
Understanding the biological mechanisms underlying the pH-dependent nature of FcRn binding, as well as the various factors influencing the affinity to FcRn, was concurrent with the arrival of the first recombinant IgG monoclonal antibodies (mAbs) and IgG Fc-fusion proteins in clinical practice. IgG Fc-FcRn became a central subject of interest for the development of these drugs for the comfort of patients and good clinical responses. In this review, we describe (i) mAb mutations close to and outside the FcRn binding site, increasing the affinity for FcRn at acidic pH and leading to enhanced mAb half-life and biodistribution, and (ii) mAb mutations increasing the affinity for FcRn at acidic and neutral pH, blocking FcRn binding and resulting, in vivo, in endogenous IgG degradation. Mutations modifying FcRn binding are discussed in association with pH-dependent modulation of antigen binding and (iii) anti-FcRn mAbs, two of the latest innovations in anti-FcRn mAbs leading to endogenous IgG depletion. We discuss the pharmacological effects, the biological consequences, and advantages of targeting IgG-FcRn interactions and their application in human therapeutics.
Keywords: FcRn; antibody engineering; monoclonal antibody; recycling; transcytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Leach J.L., Sedmak D.D., Osborne J.M., Rahill B., Lairmore M.D., Anderson C.L. Isolation from Human Placenta of the IgG Transporter, FcRn, and Localization to the Syncytiotrophoblast: Implications for Maternal-Fetal Antibody Transport. J. Immunol. 1996;157:3317–3322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

