cAMP/PKA Signaling Modulates Mitochondrial Supercomplex Organization
- PMID: 36077053
- PMCID: PMC9455794
- DOI: 10.3390/ijms23179655
cAMP/PKA Signaling Modulates Mitochondrial Supercomplex Organization
Abstract
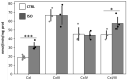
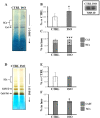
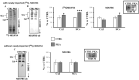
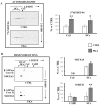
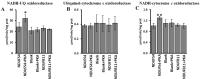
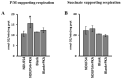
The oxidative phosphorylation (OXPHOS) system couples the transfer of electrons to oxygen with pumping of protons across the inner mitochondrial membrane, ensuring the ATP production. Evidence suggests that respiratory chain complexes may also assemble into supramolecular structures, called supercomplexes (SCs). The SCs appear to increase the efficiency/capacity of OXPHOS and reduce the reactive oxygen species (ROS) production, especially that which is produced by complex I. Studies suggest a mutual regulation between complex I and SCs, while SCs organization is important for complex I assembly/stability, complex I is involved in the supercomplex formation. Complex I is a pacemaker of the OXPHOS system, and it has been shown that the PKA-dependent phosphorylation of some of its subunits increases the activity of the complex, reducing the ROS production. In this work, using in ex vivo and in vitro models, we show that the activation of cAMP/PKA cascade resulted in an increase in SCs formation associated with an enhanced capacity of electron flux and ATP production rate. This is also associated with the phosphorylation of the NDUFS4 subunit of complex I. This aspect highlights the key role of complex I in cellular energy production.
Keywords: NDUFS4; cAMP/PKA; complex I; mitochondria; mitochondrial supercomplexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

