A C2-Domain Abscisic Acid-Related Gene, IbCAR1, Positively Enhances Salt Tolerance in Sweet Potato (Ipomoea batatas (L.) Lam.)
- PMID: 36077077
- PMCID: PMC9456122
- DOI: 10.3390/ijms23179680
A C2-Domain Abscisic Acid-Related Gene, IbCAR1, Positively Enhances Salt Tolerance in Sweet Potato (Ipomoea batatas (L.) Lam.)
Abstract
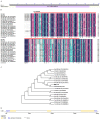
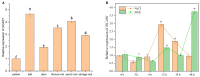
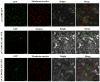
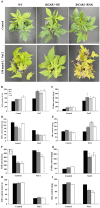
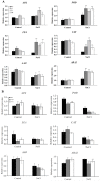
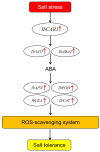
Plant C2-domain abscisic acid-related (CAR) protein family plays an important role in plant growth, abiotic stress responses, and defense regulation. In this study, we cloned the IbCAR1 by homologous cloning method from the transcriptomic data of Xuzishu8, which is a sweet potato cultivar with dark-purple flesh. This gene was expressed in all tissues of sweet potato, with the highest expression level in leaf tissue, and it could be induced by NaCl and ABA. Subcellular localization analyses indicated that IbCAR1 was localized in the nucleus and plasma membrane. The PI staining experiment revealed the distinctive root cell membrane integrity of overexpressed transgenic lines upon salt stress. Salt stress significantly increased the contents of proline, ABA, and the activity of superoxide dismutase (SOD), whereas the content of malondialdehyde (MDA) was decreased in overexpressed lines. On the contrary, RNA interference plants showed sensitivity to salt stress. Overexpression of IbCAR1 in sweet potatoes could improve the salt tolerance of plants, while the RNAi of IbCAR1 significantly increased sensitivity to salt stress in sweet potatoes. Meanwhile, the genes involved in ABA biosynthesis, stress response, and reactive oxygen species (ROS)-scavenging system were upregulated in overexpressed lines under salt stress. Taken together, these results demonstrated that IbCAR1 plays a positive role in salt tolerance by relying on the ABA signal transduction pathway, activating the ROS-scavenging system in sweet potatoes.
Keywords: IbCAR1 gene; abscisic acid; salt stress resistance; sweet potato.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Yang Y.L., Zhang X.J., Zou H.D., Chen J.Y., Wang Z.Y., Luo Z.X., Yao Z.F., Fang B.P., Huang L.F. Exploration of molecular mechanism of intraspecific cross-incompatibility in sweetpotato by transcriptome and metabolome analysis. Plant Mol. Biol. 2022;109:115–133. doi: 10.1007/s11103-022-01259-8. - DOI - PMC - PubMed
-
- Liu X.Y., Liu S.F., Zhang J., Wu Y.H., Wu W.Y., Zhang Y., Liu B.L., Tang R.M., He L.H., Li R.Z., et al. Optimization of reference genes for qRT-PCR analysis of microRNA expression under abiotic stress conditions in sweetpotato. Plant Physiol. Biochem. 2020;154:379–386. doi: 10.1016/j.plaphy.2020.06.016. - DOI - PubMed
-
- Kim S.E., Lee C.J., Ji C.Y., Kim H.S., Park S.U., Lim Y.H., Park W.S., Ahn M.J., Bian X., Xie Y., et al. Transgenic sweetpotato plants overexpressing tocopherol cyclase display enhanced alpha-tocopherol content and abiotic stress tolerance. Plant Physiol. Biochem. 2019;144:436–444. doi: 10.1016/j.plaphy.2019.09.046. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

