mPR-Specific Actions Influence Maintenance of the Blood-Brain Barrier (BBB)
- PMID: 36077089
- PMCID: PMC9456378
- DOI: 10.3390/ijms23179684
mPR-Specific Actions Influence Maintenance of the Blood-Brain Barrier (BBB)
Abstract
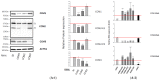
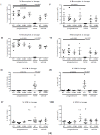
Cerebral cavernous malformations (CCMs) are characterized by abnormally dilated intracranial microvascular sinusoids that result in increased susceptibility to hemorrhagic stroke. It has been demonstrated that three CCM proteins (CCM1, CCM2, and CCM3) form the CCM signaling complex (CSC) to mediate angiogenic signaling. Disruption of the CSC will result in hemorrhagic CCMs, a consequence of compromised blood-brain barrier (BBB) integrity. Due to their characteristically incomplete penetrance, the majority of CCM mutation carriers (presumed CCM patients) are largely asymptomatic, but when symptoms occur, the disease has typically reached a clinical stage of focal hemorrhage with irreversible brain damage. We recently reported that the CSC couples both classic (nuclear; nPRs) and nonclassic (membrane; mPRs) progesterone (PRG)-receptors-mediated signaling within the CSC-mPRs-PRG (CmP) signaling network in nPR(-) breast cancer cells. In this report, we demonstrate that depletion of any of the three CCM genes or treatment with mPR-specific PRG actions (PRG/mifepristone) results in the disruption of the CmP signaling network, leading to increased permeability in the nPR(-) endothelial cells (ECs) monolayer in vitro. Finally, utilizing our in vivo hemizygous Ccm mutant mice models, we demonstrate that depletion of any of the three CCM genes, in combination with mPR-specific PRG actions, is also capable of leading to defective homeostasis of PRG in vivo and subsequent BBB disruption, allowing us to identify a specific panel of etiological blood biomarkers associated with BBB disruption. To our knowledge, this is the first report detailing the etiology to predict the occurrence of a disrupted BBB, an indication of early hemorrhagic events.
Keywords: CCM signaling complex (CSC); CSC-mPRs-PRG (CmP) signaling network; biomarkers; blood–brain barrier (BBB); cerebral cavernous malformations (CCMs); classic nuclear progesterone receptors (nPRs); endothelial cells (ECs); nonclassic membrane progesterone receptors (mPRs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Abou-Fadel J., Jiang X., Padarti A., Goswami D., Smith M., Grajeda B., Walker W., Zhang J. CCM signaling complex (CSC) is a master regulator governing homeostasis of progestins and their mediated signaling cascades. bioRxiv. 2020 doi: 10.1101/2020.06.10.145003. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

