The Role of Transcription Factor PPAR-γ in the Pathogenesis of Psoriasis, Skin Cells, and Immune Cells
- PMID: 36077103
- PMCID: PMC9456565
- DOI: 10.3390/ijms23179708
The Role of Transcription Factor PPAR-γ in the Pathogenesis of Psoriasis, Skin Cells, and Immune Cells
Abstract
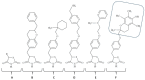
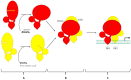
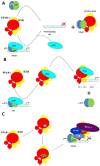
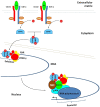
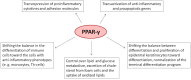
The peroxisome proliferator-activated receptor PPAR-γ is one of three PPAR nuclear receptors that act as ligand-activated transcription factors. In immune cells, the skin, and other organs, PPAR-γ regulates lipid, glucose, and amino acid metabolism. The receptor translates nutritional, pharmacological, and metabolic stimuli into the changes in gene expression. The activation of PPAR-γ promotes cell differentiation, reduces the proliferation rate, and modulates the immune response. In the skin, PPARs also contribute to the functioning of the skin barrier. Since we know that the route from identification to the registration of drugs is long and expensive, PPAR-γ agonists already approved for other diseases may also represent a high interest for psoriasis. In this review, we discuss the role of PPAR-γ in the activation, differentiation, and proliferation of skin and immune cells affected by psoriasis and in contributing to the pathogenesis of the disease. We also evaluate whether the agonists of PPAR-γ may become one of the therapeutic options to suppress the inflammatory response in lesional psoriatic skin and decrease the influence of comorbidities associated with psoriasis.
Keywords: PPAR-γ; immune cells; psoriasis; skin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Variations in the genes encoding the peroxisome proliferator-activated receptors alpha and gamma in psoriasis.Arch Dermatol Res. 2004 Jun;296(1):1-5. doi: 10.1007/s00403-004-0463-6. Epub 2004 Apr 9. Arch Dermatol Res. 2004. PMID: 15083308
-
Biological action of docosahexaenoic acid in a 3D tissue-engineered psoriatic skin model: Focus on the PPAR signaling pathway.Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Dec;1866(12):159032. doi: 10.1016/j.bbalip.2021.159032. Epub 2021 Aug 21. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 34428549
-
Peroxisome proliferator-activated receptors in cutaneous biology.Br J Dermatol. 2003 Aug;149(2):229-36. doi: 10.1046/j.1365-2133.2003.05532.x. Br J Dermatol. 2003. PMID: 12932225 Review.
-
Potential therapeutic role of peroxisome proliferator activated receptor-gamma agonists in psoriasis.Expert Opin Pharmacother. 2005 Aug;6(9):1455-61. doi: 10.1517/14656566.6.9.1455. Expert Opin Pharmacother. 2005. PMID: 16086634 Review.
-
Skin-targeted inhibition of PPAR β/δ by selective antagonists to treat PPAR β/δ-mediated psoriasis-like skin disease in vivo.PLoS One. 2012;7(5):e37097. doi: 10.1371/journal.pone.0037097. Epub 2012 May 14. PLoS One. 2012. PMID: 22606335 Free PMC article.
Cited by
-
New Insights into the Role of PPARγ in Skin Physiopathology.Biomolecules. 2024 Jun 19;14(6):728. doi: 10.3390/biom14060728. Biomolecules. 2024. PMID: 38927131 Free PMC article. Review.
-
PPARγ Agonist Rosiglitazone and Antagonist GW9662: Antihypertensive Effects on Chronic Intermittent Hypoxia-Induced Hypertension in Rats.J Cardiovasc Transl Res. 2024 Aug;17(4):803-815. doi: 10.1007/s12265-024-10499-6. Epub 2024 Feb 27. J Cardiovasc Transl Res. 2024. PMID: 38411834
-
Melatonin and Its Metabolites Can Serve as Agonists on the Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor Gamma.Int J Mol Sci. 2023 Oct 23;24(20):15496. doi: 10.3390/ijms242015496. Int J Mol Sci. 2023. PMID: 37895177 Free PMC article.
-
Comprehensive transcriptional analysis of pig facial skin development.PeerJ. 2023 Aug 28;11:e15955. doi: 10.7717/peerj.15955. eCollection 2023. PeerJ. 2023. PMID: 37663277 Free PMC article.
-
Integrating network pharmacology, molecular docking, and experimental validation to reveal the mechanism of Radix Rehmanniae in psoriasis.Medicine (Baltimore). 2024 Oct 25;103(43):e40211. doi: 10.1097/MD.0000000000040211. Medicine (Baltimore). 2024. PMID: 39470475 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

