Balanced Free Essential Amino Acids and Resistance Exercise Training Synergistically Improve Dexamethasone-Induced Impairments in Muscle Strength, Endurance, and Insulin Sensitivity in Mice
- PMID: 36077132
- PMCID: PMC9456044
- DOI: 10.3390/ijms23179735
Balanced Free Essential Amino Acids and Resistance Exercise Training Synergistically Improve Dexamethasone-Induced Impairments in Muscle Strength, Endurance, and Insulin Sensitivity in Mice
Abstract
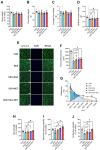
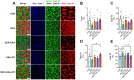
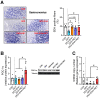
Our previous study shows that an essential amino acid (EAA)-enriched diet attenuates dexamethasone (DEX)-induced declines in muscle mass and strength, as well as insulin sensitivity, but does not affect endurance. In the present study, we hypothesized that the beneficial effects will be synergized by adding resistance exercise training (RET) to EAA, and diet-free EAA would improve endurance. To test hypotheses, mice were randomized into the following four groups: control, EAA, RET, and EAA+RET. All mice except the control were subjected to DEX treatment. We evaluated the cumulative rate of myofibrillar protein synthesis (MPS) using 2H2O labeling and mass spectrometry. Neuromuscular junction (NMJ) stability, mitochondrial contents, and molecular signaling were demonstrated in skeletal muscle. Insulin sensitivity and glucose metabolism using 13C6-glucose tracing during oral glucose tolerance tests were analyzed. We found that EAA and RET synergistically improve muscle mass and/or strength, and endurance capacity, as well as insulin sensitivity, and glucose metabolism in DEX-treated muscle. These improvements are accomplished, in part, through improvements in myofibrillar protein synthesis, NMJ, fiber type preservation, and/or mitochondrial biogenesis. In conclusion, free EAA supplementation, particularly when combined with RET, can serve as an effective means that counteracts the adverse effects on muscle of DEX that are found frequently in clinical settings.
Keywords: dexamethasone; essential amino acids; glucose metabolic flux; mitochondrial biogenesis; muscle atrophy; neuromuscular junction stability; physical performance; protein turnover; resistance exercise training.
Conflict of interest statement
I.-Y.K., S.H.P. and J.Y.J. are stockholders of Myocare. Inc., and Robert Wolfe is a shareholder in Essential Blends, LLC, and the Amino Company, LLC. All others have no potential conflict of interest.
Figures



References
-
- Lee H., Kim Y.I., Nirmala F.S., Jeong H.Y., Seo H.-D., Ha T.Y., Jung C.H., Ahn J. Chrysanthemum zawadskil Herbich attenuates dexamethasone-induced muscle atrophy through the regulation of proteostasis and mitochondrial function. Biomed. Pharmacother. 2021;136:111226. doi: 10.1016/j.biopha.2021.111226. - DOI - PubMed
-
- Kim Y., Park S., Lee J., Jang J., Jung J., Koh J.-H., Choi C.S., Wolfe R.R., Kim I.-Y. Essential Amino Acid-Enriched Diet Alleviates Dexamethasone-Induced Loss of Muscle Mass and Function through Stimulation of Myofibrillar Protein Synthesis and Improves Glucose Metabolism in Mice. Metabolites. 2022;12:84. doi: 10.3390/metabo12010084. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

