Post-COVID-19 Parkinsonism and Parkinson's Disease Pathogenesis: The Exosomal Cargo Hypothesis
- PMID: 36077138
- PMCID: PMC9456372
- DOI: 10.3390/ijms23179739
Post-COVID-19 Parkinsonism and Parkinson's Disease Pathogenesis: The Exosomal Cargo Hypothesis
Abstract
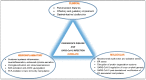
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease after Alzheimer's disease, globally. Dopaminergic neuron degeneration in substantia nigra pars compacta and aggregation of misfolded alpha-synuclein are the PD hallmarks, accompanied by motor and non-motor symptoms. Several viruses have been linked to the appearance of a post-infection parkinsonian phenotype. Coronavirus disease 2019 (COVID-19), caused by emerging severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, has evolved from a novel pneumonia to a multifaceted syndrome with multiple clinical manifestations, among which neurological sequalae appear insidious and potentially long-lasting. Exosomes are extracellular nanovesicles bearing a complex cargo of active biomolecules and playing crucial roles in intercellular communication under pathophysiological conditions. Exosomes constitute a reliable route for misfolded protein transmission, contributing to PD pathogenesis and diagnosis. Herein, we summarize recent evidence suggesting that SARS-CoV-2 infection shares numerous clinical manifestations and inflammatory and molecular pathways with PD. We carry on hypothesizing that these similarities may be reflected in exosomal cargo modulated by the virus in correlation with disease severity. Travelling from the periphery to the brain, SARS-CoV-2-related exosomal cargo contains SARS-CoV-2 RNA, viral proteins, inflammatory mediators, and modified host proteins that could operate as promoters of neurodegenerative and neuroinflammatory cascades, potentially leading to a future parkinsonism and PD development.
Keywords: Parkinson’s disease; SARS-CoV-2; alpha-synuclein; exosomes; inflammation; neurodegeneration; neuroinflammation; parkinsonism; post-COVID-19; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Keener A.M., Bordelon Y.M. Parkinsonism. Proc. Semin. Neurol. 2016;36:330–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

